AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2026-01-14 View volume: 171
Background
Ovarian cancer remains one of the deadliest gynecologic malignancies. While the overall 5-year relative survival is approximately 52% in the United States, it drops to around 32% once diagnosed at a distant stage, underscoring the clinical burden of late presentation. Recurrence and chemotherapy resistance are major clinical dilemmas. Ovarian cancer stem-like cells (OCSLCs) are key drivers of resistance, yet the regulatory role and mechanism of peptidylarginine deiminase 1 (PAD1) in OCSLCs have not been fully defined—highlighting the need for actionable mechanistic targets to break therapeutic bottlenecks.
Literature Overview
This study, published in Advanced Science (doi: 10.1002/advs.202501014), investigates PAD1 function in OCSLCs. Using RNA-seq, co-immunoprecipitation, xenograft models, and clinical sample validation (33 ovarian cancer tissues and 33 ovarian cyst tissues), the authors identify the “PAD1–AKT2/R202 citrullination–CEBPβ” axis as a regulatory mechanism supporting OCSLC stemness. They further show that PAD1-axis activation is enriched in cisplatin-resistant OVCAR3-CisR cells, and that pharmacological PAD1 inhibition can re-sensitize resistant cells to cisplatin and reduce tumor burden in vivo. A site-specific Cit202 antibody customized at AtaGenix supports the detection of AKT2/R202 citrullination in key validation workflows, enabling robust modification readouts for mechanism interrogation and biomarker-oriented exploration.
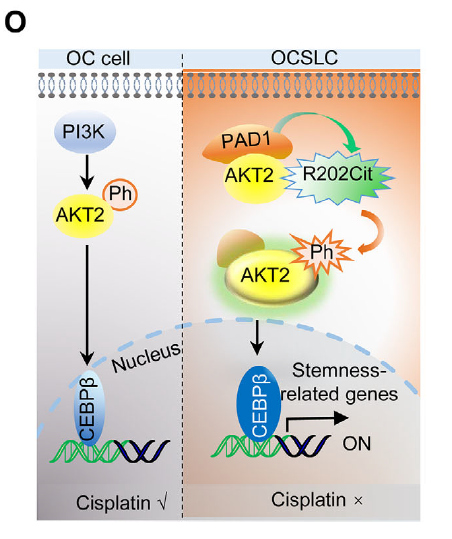
Fig. 1: Mechanistic model of PAD1 regulating OCSLCs
Core Mechanism: Cascade Activation of the Signaling Axis
PAD1 is highly expressed in OCSLCs (CD133⁺ sorted cells) and specifically binds to the kinase domain (152-481aa) of AKT2, catalyzing its citrullination at Arg202. This modification maintains the exposure of AKT2 phosphorylation sites (S474/T309), promoting its kinase activity to activate the PI3K-AKT pathway. Consequently, the transcription factor CEBPβ is upregulated, ultimately activating stemness genes such as CD133 and SOX2 to sustain OCSLC self-renewal and tumor-initiating capacity.
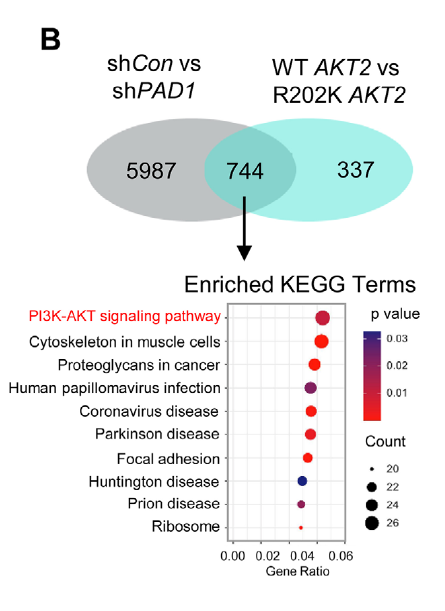
Fig. 2: Validation of PAD1-AKT2 interaction
Detection Support from AtaGenix-Customized Antibody
The Cit202 antibody, customized at AtaGenix, provides a reliable, site-specific readout for mechanistic validation. It is designed to recognize AKT2 Arg202 citrullination while minimizing cross-reactivity with AKT homologs or unmodified AKT2, enabling clear interpretation in workflows such as co-immunoprecipitation and immunohistochemistry. In the reported study context, Cit202 staining and signal dynamics support direct evidence for “PAD1 regulates AKT2/R202 citrullination” and facilitate downstream exploration of modification-linked phenotypes and biomarker potential.
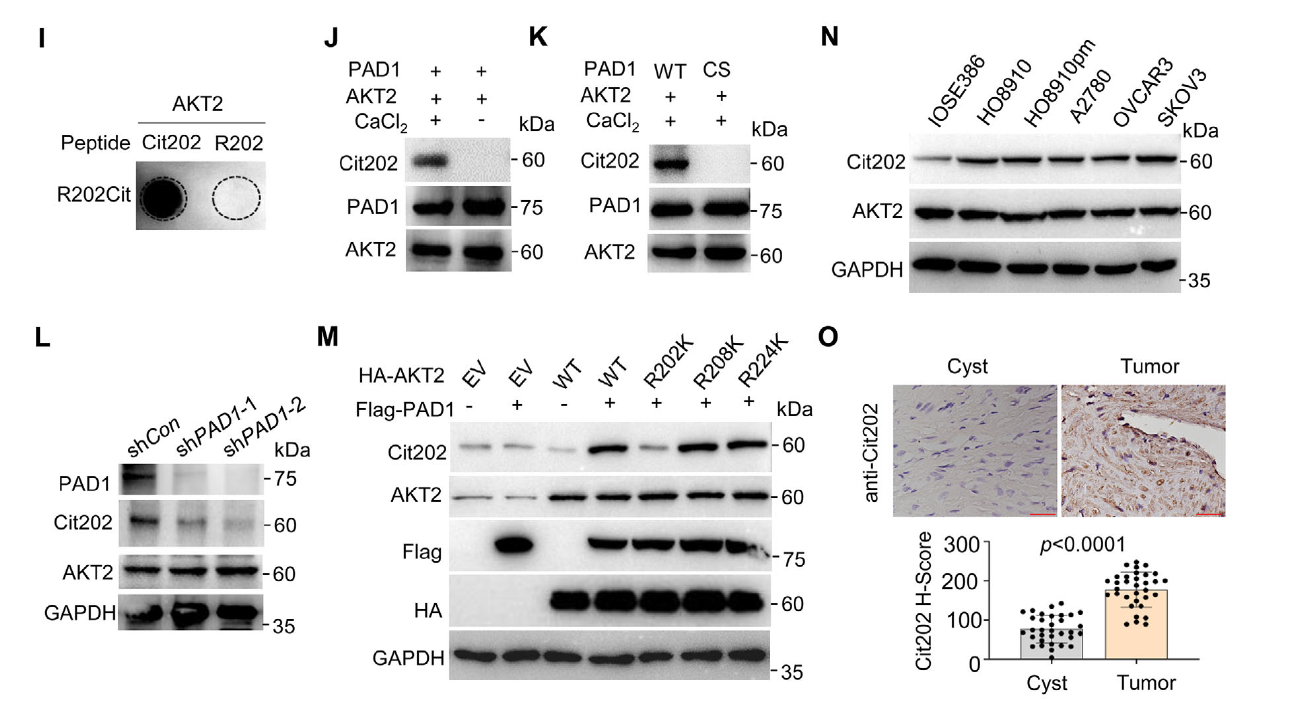
Fig. 3: Results of Cit202 antibody
Conclusion
In summary, this study supports the “PAD1–AKT2/R202–CEBPβ” axis as a mechanistic driver of OCSLC-associated stemness and cisplatin resistance. The Cit202 antibody, customized at AtaGenix, provides practical tool support for detecting AKT2 R202 citrullination in key validation assays. In preclinical models, pharmacological PAD1 inhibition combined with cisplatin suggests a stemness-oriented intervention strategy for overcoming resistance and motivates further translational evaluation.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

