AtaGenix Laboratories
AtaGenix Laboratories
Hybridoma antibody technology produces monoclonal antibodies but faces challenges like instability and the need for liquid nitrogen preservation. Hybridoma sequencing enables recombinant expression, ensuring stability and preventing biological contamination.
AtaGenix offers high-accuracy hybridoma sequencing and recombinant antibody expression, achieving 200-500 mg/L yields in 2 weeks with the proprietary pATX3.0 vector.
Flexible Sample Options
Accepts both cultured cells and frozen cell lines for seamless processing
Rapid Turnaround
Delivers results within just 2 weeks, ensuring efficiency
Comprehensive Analysis
Offers variable region and full-length sequencing, with detailed reports including CDR region analysis
At AtaGenix, we pride ourselves on over 10 years of expertise in delivering custom hybridoma monoclonal antibody services. Our process is designed to meet the highest standards of quality and efficiency, ensuring your research and therapeutic projects are supported by the best monoclonal antibodies available.

Accelerate cell line projects by over 50% with real-time, high-resolution imaging confirming monoclonality using VIPS™ technology.

Secure your investments with stable cell line development services achieving yields exceeding 7g/L.

Access royalty-free cell lines such as CHO-1, CHO-S, CHO-DG44, HEK293, or your preferred choice.

Reduce production risks with extensive early testing of antibody candidates.

Fast cell line and protocol transfer to CMO partners for cGMP bioproduction.
500+ monoclonal antibodies developed, 100+ stable cell lines generated, 28+ years of expertise.
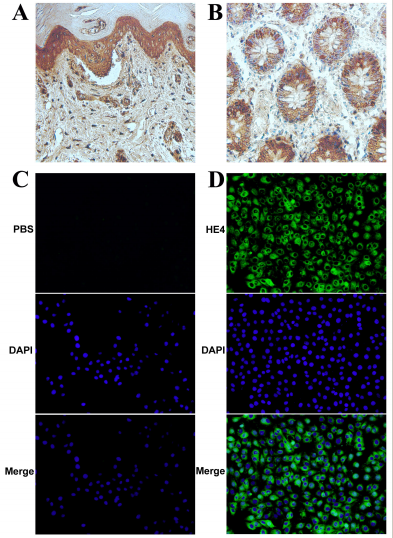
A 2017 study developed a high-affinity anti-HE4 monoclonal antibody (9C3) using AtaGenix’s advanced mammalian expression and hybridoma platforms. This breakthrough enhances ovarian cancer diagnostics by targeting the HE4 biomarker with high specificity, offering potential for early detection and therapy.
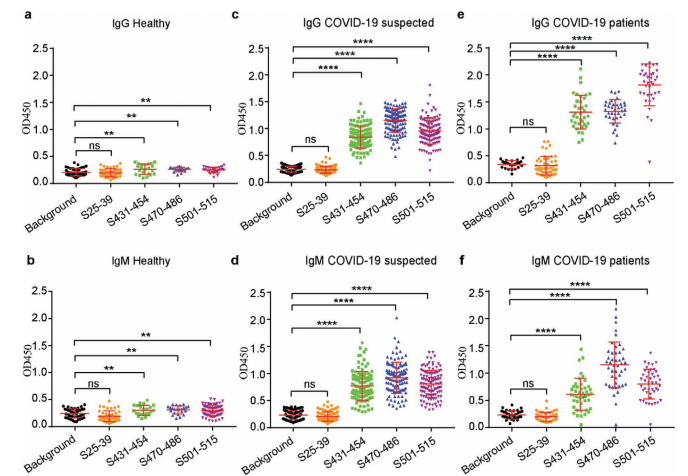
This article highlights a 2021 Small Methods study that identified immunogenic peptides in the SARS-CoV-2 spike protein and isolated neutralizing monoclonal antibodies from COVID-19 patients. The research pinpointed key RBM epitopes (S431-454, S470-486, S501-515), with S470-486 emerging as an immunodominant target for diagnostics and therapeutics. Using phage display and ScFv libraries, the study validated epitope mapping and antibody functionality. Supported by AtaGenix’s expertise in phage display, protein expression, and assay development, this breakthrough offers a scalable approach for combating emerging viral pathogens, updated as of August 24, 2025.