AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-09-29 View volume: 719
Intrahepatic cholangiocarcinoma (ICC) is a highly aggressive malignancy of the biliary system, with its incidence steadily increasing in recent years. Particularly in the context of excessive AKT pathway activation, ICC not only exhibits more aggressive biological features but also shows resistance to chemotherapy and immunotherapy. Ferroptosis, an iron-dependent form of cell death, has emerged as a potential breakthrough for cancer therapy. However, some ICC cells evade ferroptosis through metabolic reprogramming and signal pathway regulation, limiting treatment efficacy.
In May 2025, researchers from Huazhong University of Science and Technology, Tongji Medical College, and the First Affiliated Hospital of Soochow University published a study in Cancer Communications titled “Simvastatin Overcomes the pPCK1-pLDHA-SPRINGlac Axis-Mediated Ferroptosis and Chemoresistance in AKT-Hyperactivated Intrahepatic Cholangiocarcinoma.” The study focused on the role of the pPCK1-pLDHA-SPRINGlac axis in ferroptosis resistance and proposed that the commonly used lipid-lowering drug Simvastatin could restore ferroptosis sensitivity, offering new therapeutic insights for ICC.
1. Core Mechanism: The Full Chain of Resistance Mediated by the pPCK1-pLDHA-SPRINGlac Axis
1.1 Key Markers and Regulatory Axis Activation
Phosphorylated phosphoenolpyruvate carboxykinase 1 (pPCK1, Ser90 phosphorylation) is a core marker of chemoresistance in AKT-hyperactivated ICC. AKT activation induces pPCK1 expression, detaching it from its traditional gluconeogenic function and redirecting it to regulate glycolysis and lipid metabolism, thus activating the pPCK1-pLDHA-SPRINGlac axis.
1.2 Axis Function and MVA Pathway Activation
This axis drives chemoresistance through a process of "molecular interaction → metabolic reprogramming," specifically by enabling pPCK1 to bind to lactate dehydrogenase A (LDHA) and phosphorylate its T248 site, thereby enhancing lactate production. The resulting lactate activates lysine acetyltransferase 7 (KAT7), which mediates the lactylation of SPRING protein at K82 (SPRINGlac). SPRINGlac promotes the transport of the SCAP-SREBP complex to the Golgi apparatus, thereby activating the mevalonate (MVA) pathway. Its downstream products, coenzyme Q10 (CoQ10H2) and menaquinone-4 (MK4), inhibit ferroptosis by scavenging lipid peroxides and also suppress immune responses, ultimately leading to ICC resistance to chemotherapy and immunotherapy (CIT).
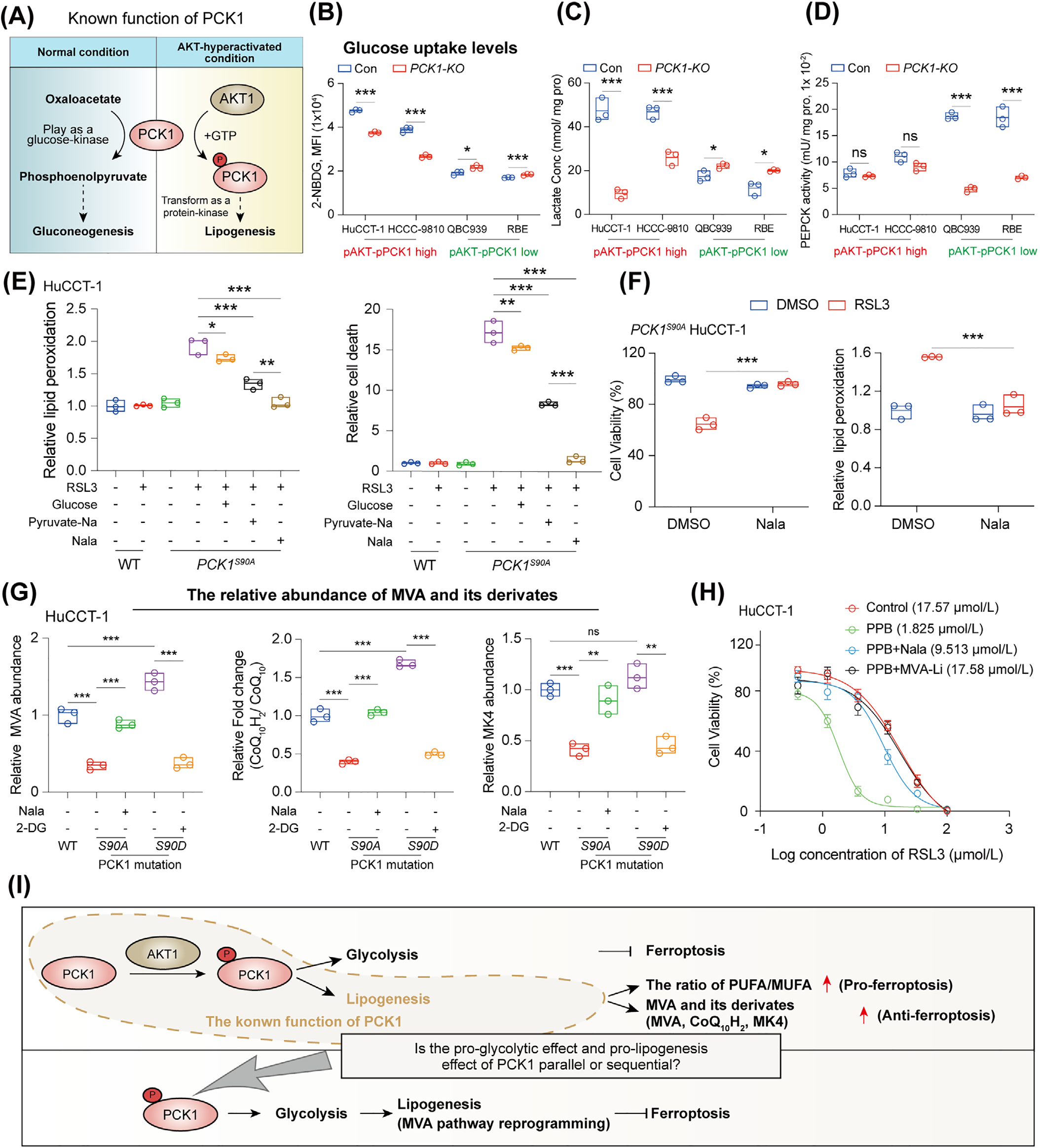
Figure 1. pPCK1 Regulates Lactate Metabolism and MVA Flux Reprogramming, Contributing to Ferroptosis Resistance in ICC.
1.3 Potential of Repurposing an Old Drug: Simvastatin
Simvastatin, by inhibiting the key enzyme HMGCR in the MVA pathway, can block the downstream effects of the pPCK1-pLDHA-SPRINGlac axis, reversing ferroptosis resistance and enhancing CIT efficacy. This provides a new strategy for treating resistant ICC.
2. Multi-Dimensional Validation: From Basic to Clinical Evidence
2.1 Basic Validation of Core Molecules and Mechanisms
2.1.1 Molecular Screening and Localization
Through an in vivo screen using an sgRNA library targeting 2865 metabolic genes, PCK1 was identified as a primary regulatory gene for CIT resistance. IP-MS identified Ser90 as the key phosphorylation site of pPCK1, with a PCK1S90A mutation decreasing ICC cell resistance by 70%.
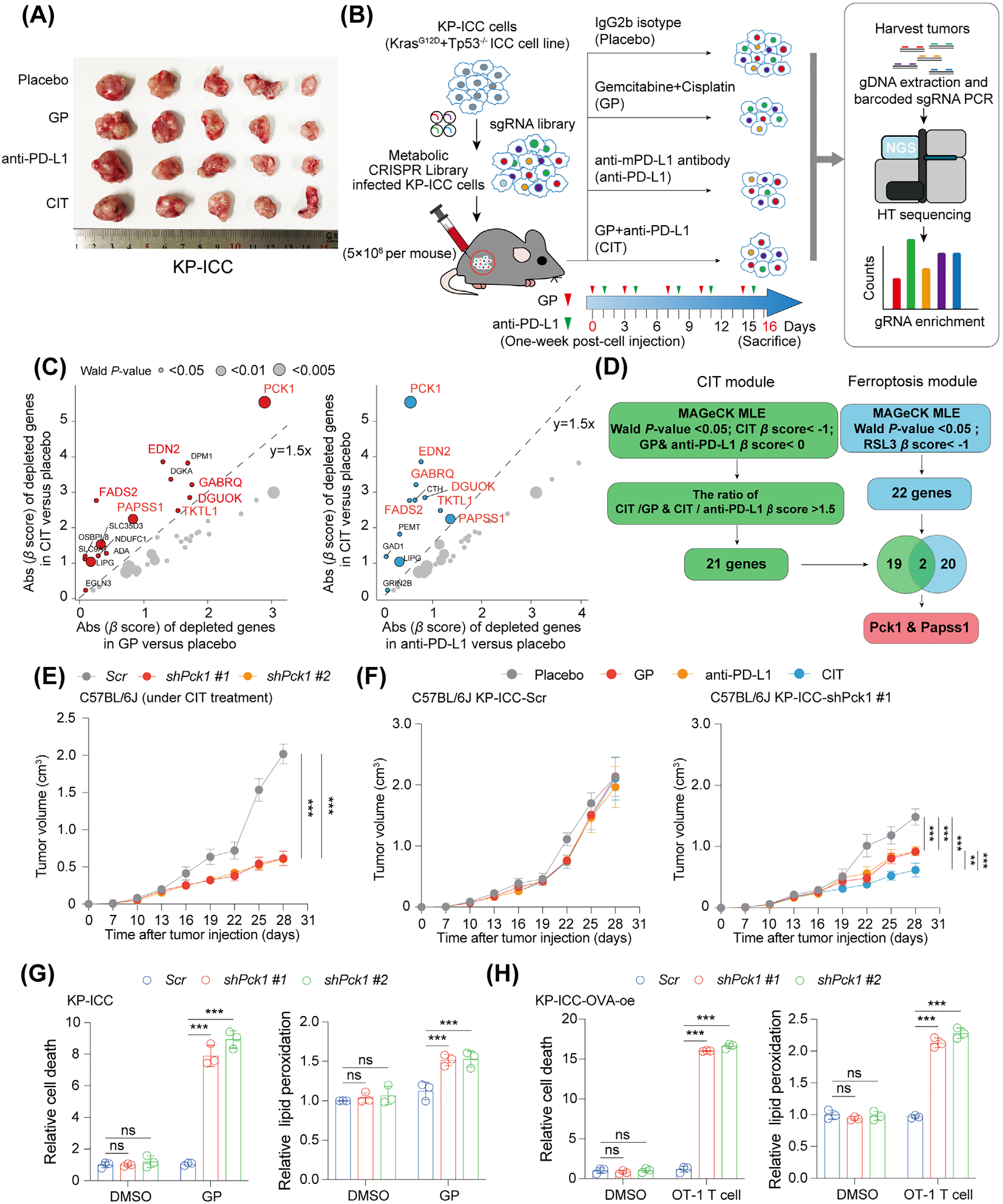
Figure 2. In Vivo Metabolic Screening Reveals Ferroptosis Regulators in ICC under CIT Treatment.
2.1.2 Cellular and Animal Model Validation
Cellular Level: The PCK1S90A mutation made ICC cells 3.2 times more sensitive to ferroptosis inducer RSL3. Supplementing with MVA precursors (MVA-Li) restored the resistant phenotype.
Animal Level: Suppression of pPCK1 expression increased the CIT response rate in AKT-hyperactivated ICC xenografts from 28% to 65%, and raised tumour lipid peroxidation levels by 2.8-fold.
2.2 Clinical Correlation Verification
In an analysis of 126 AKT-hyperactivated ICC patients undergoing CIT, those with high pPCK1 expression (IHC score ≥ 3) had a median progression-free survival of 5.3 months, significantly shorter than the 9.5 months in the low-expression group (P < 0.001). This confirmed that pPCK1 and its regulatory axis are directly related to clinical chemoresistance.
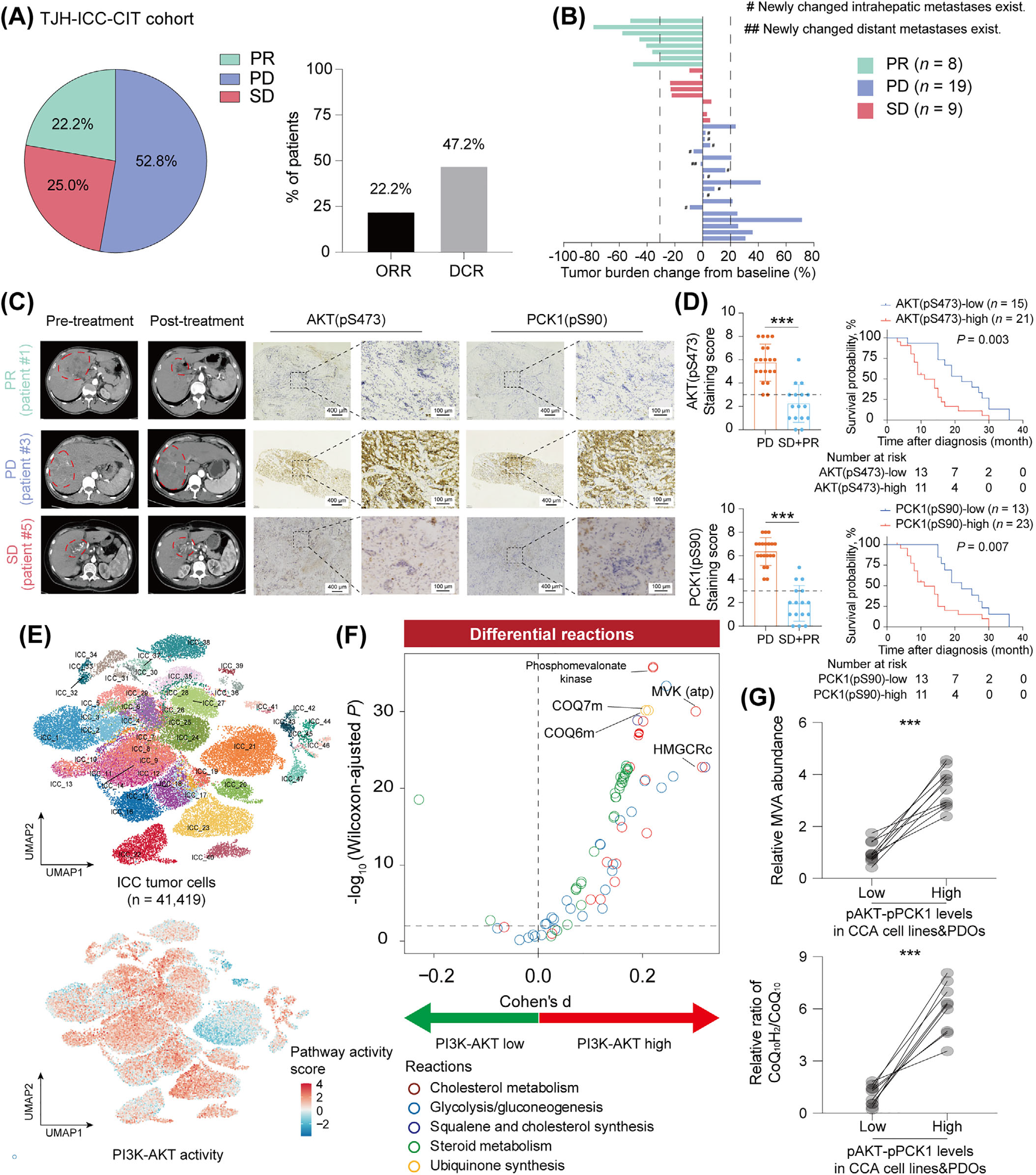
Figure 3. Prognostic Correlation of pPCK1 in AKT-Overactivated ICC Patients Undergoing CIT.
This study addresses the challenge of CIT resistance in AKT-hyperactivated ICC by elucidating the core mechanism of ferroptosis resistance mediated by the pPCK1-pLDHA-SPRINGlac axis. It also demonstrates that Simvastatin can target and block this axis to reverse resistance. The evidence chain, from sgRNA screening to cellular/animal experiments and clinical sample validation, not only establishes pPCK1's value as a biomarker for resistance but also provides a novel therapeutic direction for resistant ICC using an existing drug.
AtaGenix specially developed anti-PCK1 pS90 antibody, which exhibits high specificity for the Ser90 phosphorylation site of PCK1, was a key tool in overcoming this technical bottleneck and directly supported the discovery of the "pPCK1-pLDHA-SPRINGlac axis-mediated chemoresistance" mechanism.
AtaGenix technologies have helped researchers overcome numerous research bottlenecks, leading to significant discoveries. By the end of August 2025, over 400 publications have cited AtaGenix one-stop protein and antibody development services. Looking forward, AtaGenix will continue to support scientific research, driving technological innovation and breakthroughs.
For more information, please visit www.atagenix.com.
作为行业专家是否有错漏
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

