AtaGenix Laboratories
AtaGenix Laboratories
Are long timelines, inconsistent results, and low yields slowing down your monoclonal antibody development? AtaGenix accelerates your journey with our advanced Verified In-Situ Plate Seeding (VIPS™) technology, cutting development time by over 50% while ensuring clonality reports critical for IND submission. Our IP-free, royalty-free cell lines are designed for stability and high productivity, achieving yields exceeding 7g/L. With decades of expertise and a track record of generating 100+ stable cell lines, we provide a robust platform optimized for developability. Boost your research and bioproduction with AtaGenix’s innovative solutions.
AtaGenix streamlines stable cell line generation with high-efficiency solutions for biopharmaceutical development. Using VIPS™ technology (optional), we accelerate projects by 50%, ensuring monoclonality with high-resolution imaging. Our process delivers yields exceeding 7g/L, supporting long-term stability. We offer XtenCHOTM IP-free cell line, HEK293 and developability-focused testing to minimize production risks. With 15+ years of expertise, 5000+ de novo monoclonal antibodies developed, and 100+ stable cell lines generated, we ensure a seamless transfer to scale up production.

Accelerate cell line projects by over 50% with real-time, high-resolution imaging confirming monoclonality using VIPS™ technology.

Secure your investments with stable cell line development services achieving yields exceeding 7g/L.

Access royalty-free cell lines such as CHO-1, CHO-S, CHO-DG44, HEK293, or your preferred choice.

Reduce production risks with extensive early testing of antibody candidates.

Fast cell line and protocol transfer to CMO partners for cGMP bioproduction.
500+ monoclonal antibodies developed, 100+ stable cell lines generated, 28+ years of expertise.
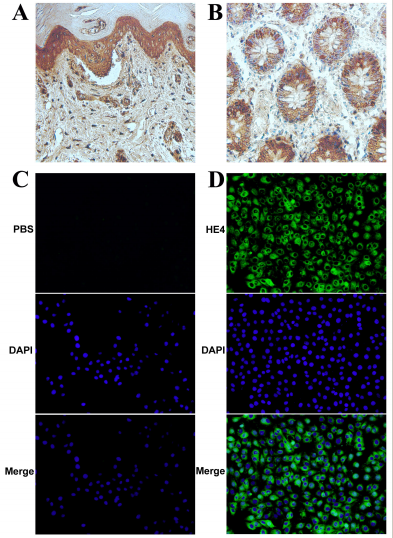
A 2017 study developed a high-affinity anti-HE4 monoclonal antibody (9C3) using AtaGenix’s advanced mammalian expression and hybridoma platforms. This breakthrough enhances ovarian cancer diagnostics by targeting the HE4 biomarker with high specificity, offering potential for early detection and therapy.
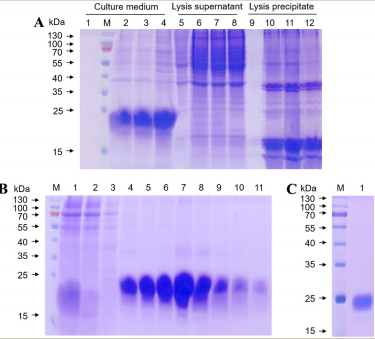
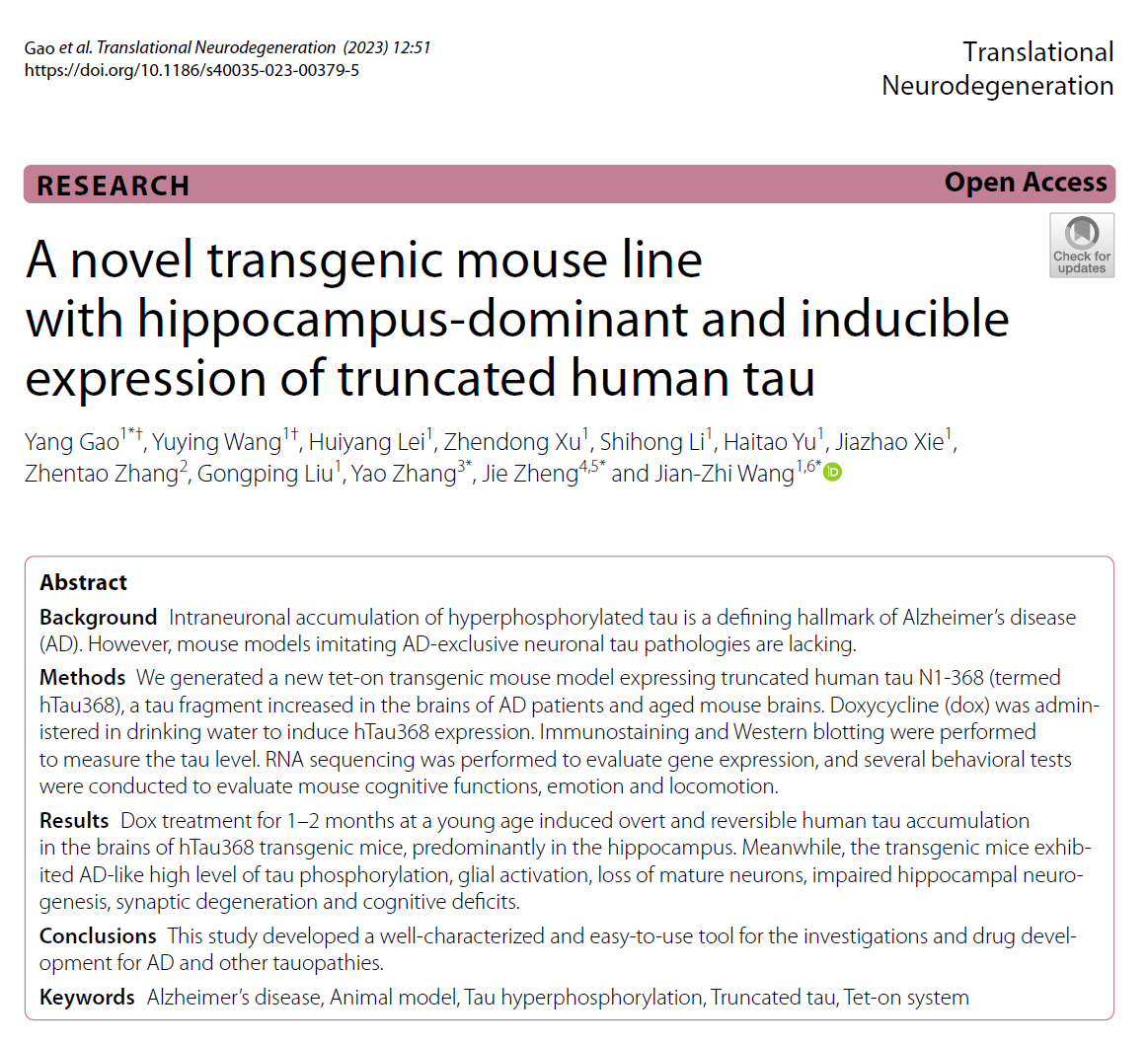
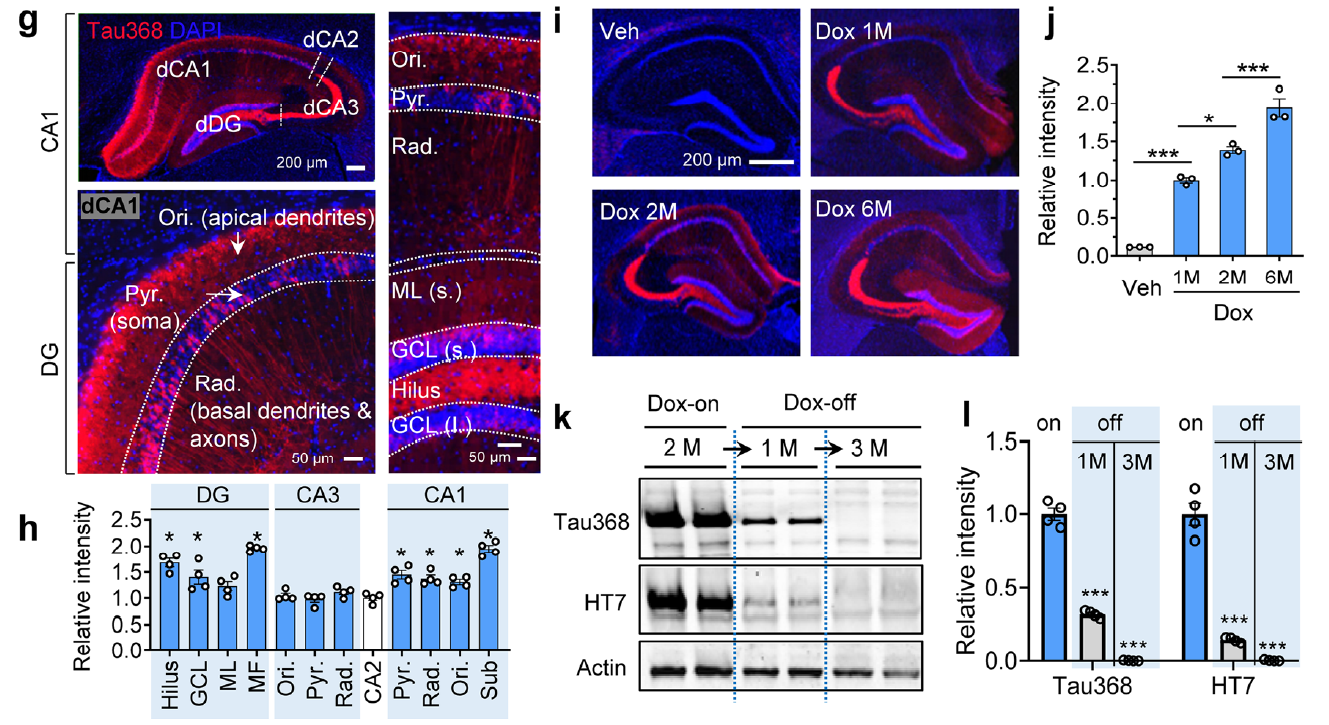
AtaGenix provides highly specific anti-tauN368 monoclonal antibodies to support Alzheimer’s disease research using the hTau368 transgenic mouse model. These antibodies enable precise detection of truncated tau fragments in Western blot and immunofluorescence experiments, validating tau accumulation, phosphorylation, and associated cognitive deficits in the hippocampus. With exceptional specificity and stability, AtaGenix’s solutions empower researchers to explore tau pathology mechanisms and advance tau-targeted therapeutic development.