AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-09-12 View volume: 616
Hepatocellular Carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide, with a 5-year survival rate for advanced patients below 20%. Current treatments, such as surgical resection and targeted therapies, provide relief but face challenges like high recurrence rates and drug resistance. There is an urgent need to develop immunotherapies that enhance tumor-specific T-cell infiltration. Personalized neoantigen mRNA vaccines, which target tumor mutation-derived antigens, hold promise for eliciting robust immune responses. However, the preferential liver distribution of mRNA delivery systems may cause liver damage, making the design of spleen-targeting lipid nanoparticles (LNPs) a critical research focus to improve efficacy and safety.
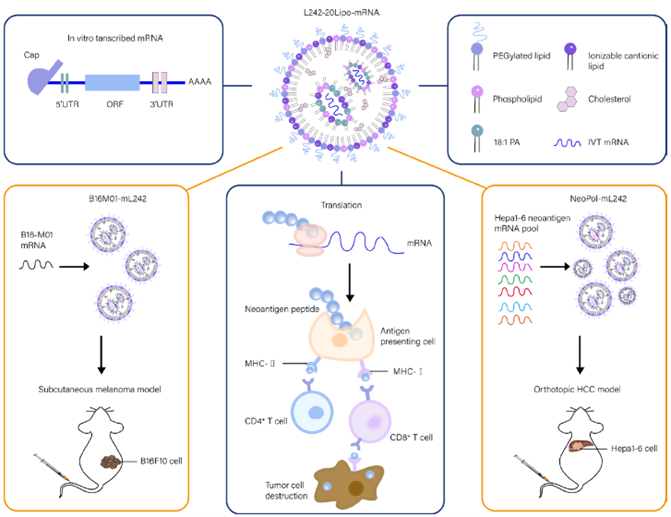
Figure 1. L242-20Lipo delivers personalized neoantigen mRNA in vivo
Screening and Optimization of LNPs for Spleen-Targeted mRNA Delivery
In the initial phase, a library of ionizable lipids was synthesized, identifying 25 candidates. LNPs formulated with 18:1 PA were tested for in vivo GFP mRNA delivery, with L221 and L242 showing the highest transfection efficiency. Further optimization of the L242-20Lipo molar ratio demonstrated significant spleen accumulation and minimal liver distribution in luciferase mRNA delivery experiments, with a spleen-to-liver signal ratio 4.5 times higher than controls. Physicochemical characterization confirmed that surface charge modulation enhanced spleen selectivity (Fig. 2E-H), laying the foundation for subsequent immune targeting.
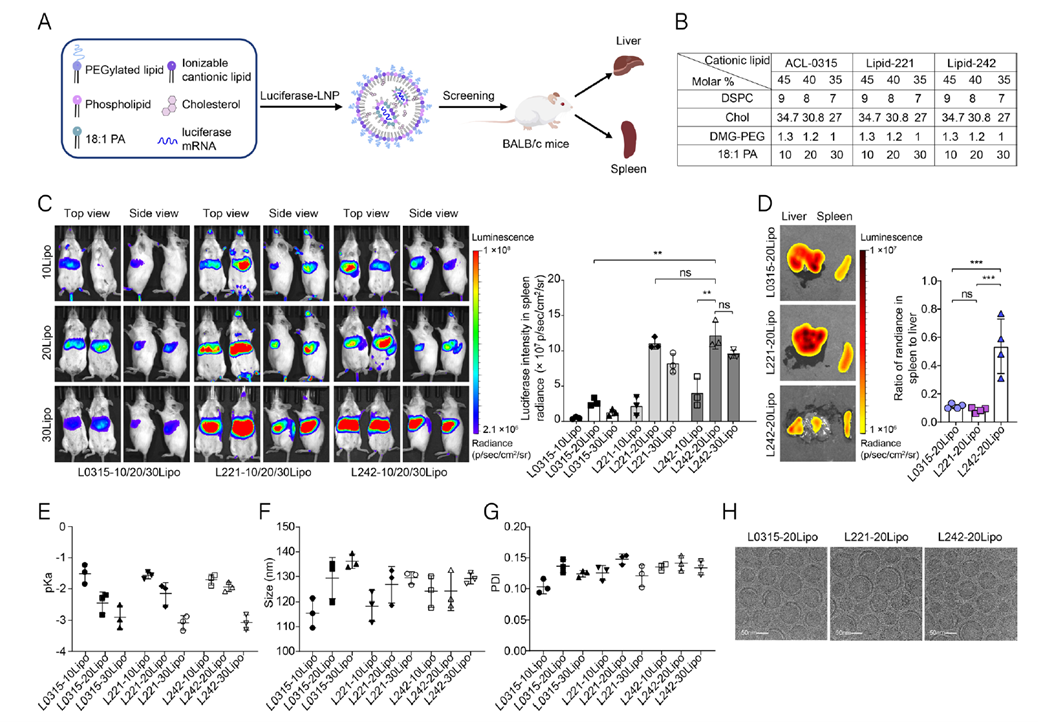
Figure 2. Screening of spleen-targeting lipid nanoparticles
Impact of Spleen-Targeting LNPs on Dendritic Cells (DCs)
To evaluate the effect of spleen-targeting LNPs on dendritic cells (DCs), mRNA encoding Cre recombinase was delivered in Ai14 reporter mice, with tdTomato expression used to track transfected cell subsets. Results showed that Cre-L242-20Lipo achieved a 27% uptake rate in DCs, 3.4 times higher than the control L0315-20Lipo. Dose-dependent GFP mRNA delivery further confirmed its DC-targeting advantage. After delivering neoantigen mRNA (Hepa-M01-mRNA), CD40 and CD86 expression in DCs increased by 7.5- and 5.7-fold, respectively, while ELISA assays detected significantly elevated IL-12 and TNF-α secretion. These findings indicate that L242-20Lipo effectively promotes antigen uptake and DC activation, establishing a foundation for neoantigen vaccines to induce robust immune responses.
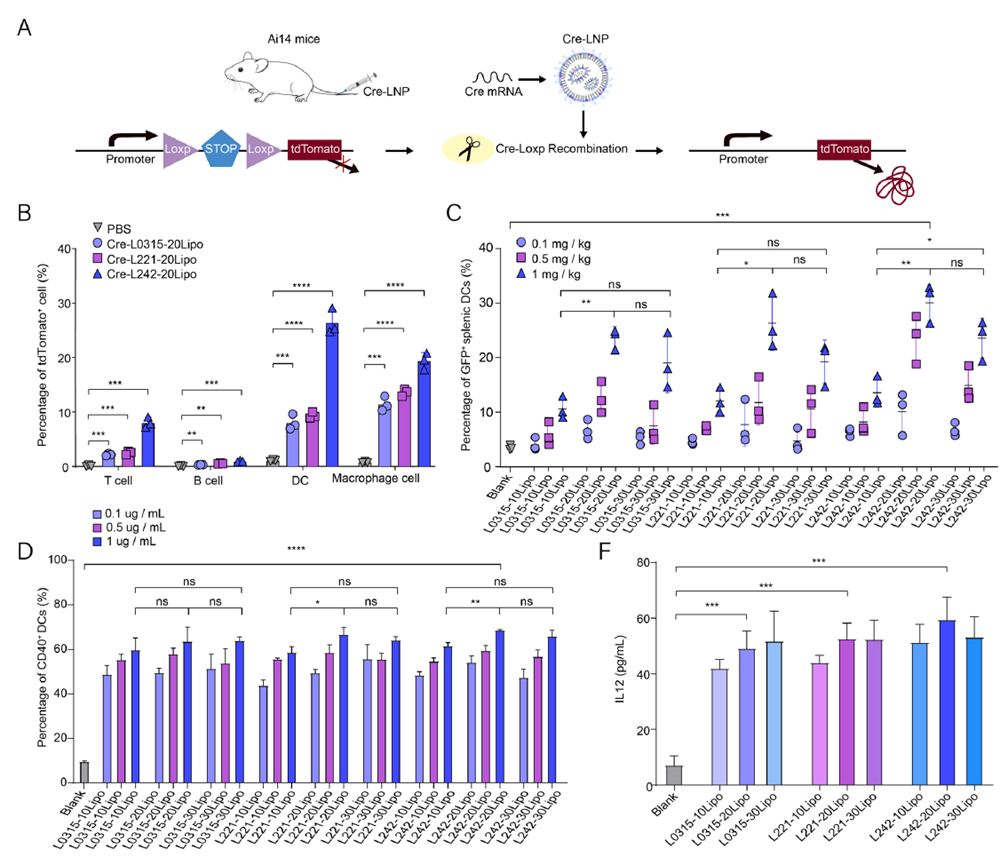
Figure 3. Exploration of key cells in mRNA presentation
Validation of B16M01-mL242 in the B16F10 Tumor Model
In the B16F10 melanoma model, the research team administered the B16M01-mL242 vaccine at a dose of 0.5 mg/kg in three vaccinations, successfully inducing T-cell activation in the spleen and lymph nodes. This approach significantly enhanced immune responses, resulting in marked reductions in tumor volume and weight and prolonged mouse survival. Detailed analysis revealed that tumor-infiltrating lymphocytes (TILs) in vaccinated mice exhibited strong IFN-γ secretion by CD8+ T cells, demonstrating the vaccine’s effectiveness in inducing anti-tumor immune responses against melanoma.
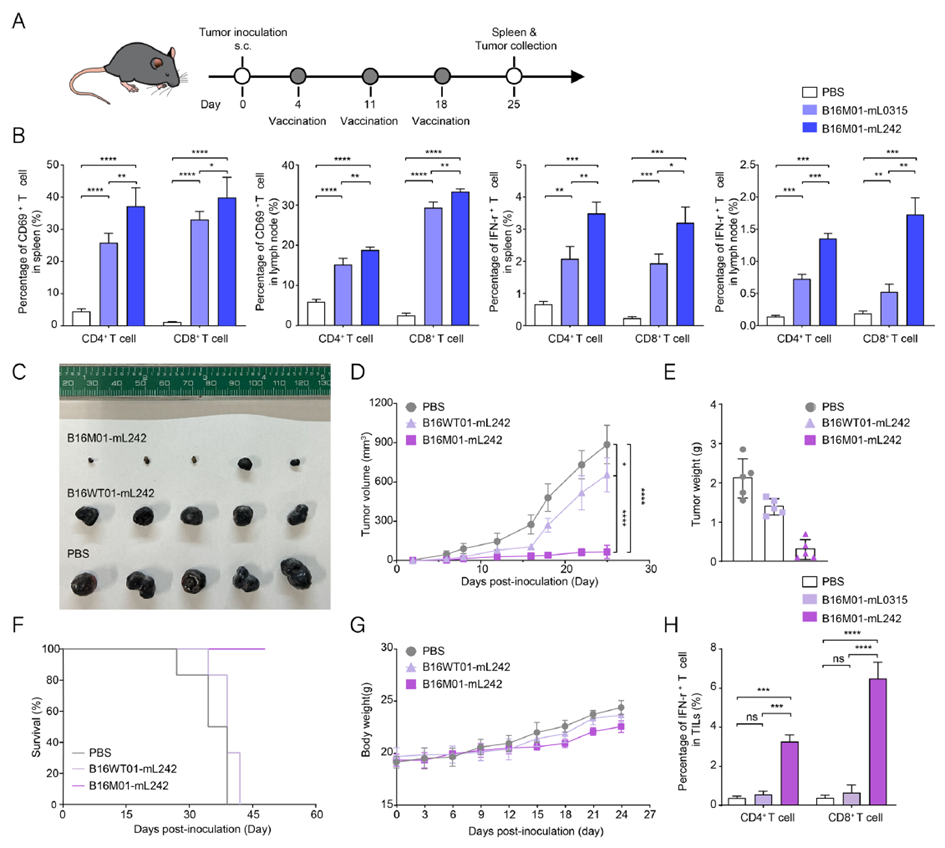
Figure 4. T-cell responses and anti-tumor efficacy of B16M01-mL242
Anti-Tumor Immunity of NeoPol-mL242 in an Orthotopic Hepatocellular Carcinoma Model
To assess the anti-tumor immunity of NeoPol-mL242 in an orthotopic HCC model, the research team designed NeoPol to include seven neoantigens (Hepa-M01 to M07). Mice received three vaccinations, and tumor progression was monitored via in vivo imaging. Results showed that the NeoPol-mL242 group had approximately 90% lower tumor burden compared to controls, with the 0.5 mg/kg group exhibiting significant survival prolongation and stable body weight. Further analysis revealed higher CD8+ T-cell infiltration in tumors, with Ki67 and TUNEL assays indicating significantly reduced tumor cell proliferation and increased apoptosis. These findings demonstrate that NeoPol-mL242 effectively activates anti-tumor immunity and inhibits HCC growth.
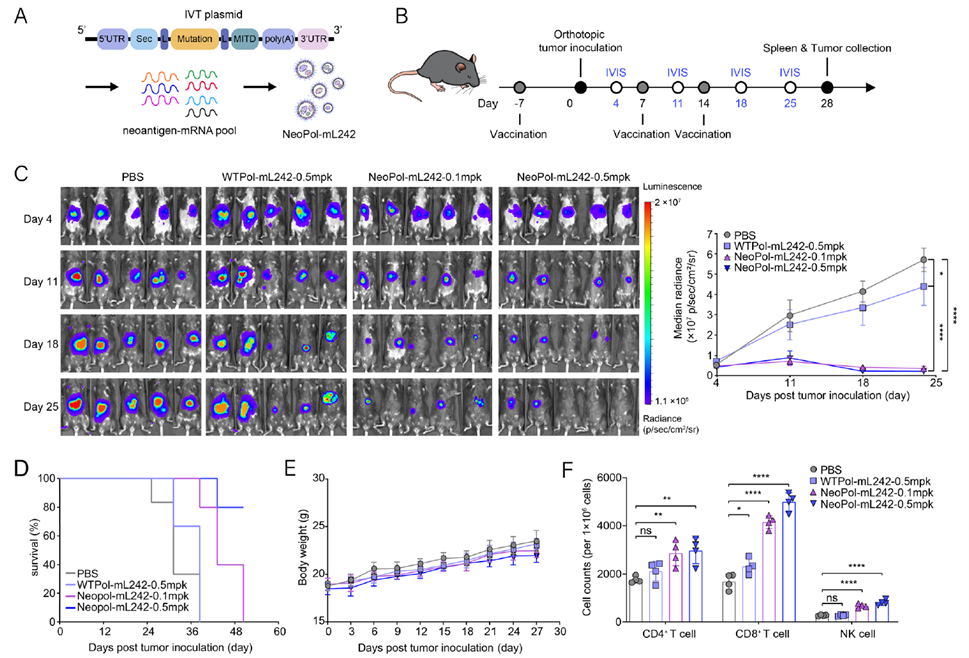
Figure 5. Immune specificity of the NeoPol-mL242 vaccine
Immune Specificity of the NeoPol-mL242 Vaccine
Based on these results, the study further evaluated the immune specificity of the NeoPol-mL242 vaccine. Peptide-MHC tetramer staining analyzed neoantigen-specific CD8+ TILs and central memory CD8+ T cells (TCMs, CD44+ CD62L+). The vaccine group showed a higher proportion of neoantigen-specific CD8+ T cells and significantly increased CD8+ TCMs. The Anti-mouse MHC-Tetramer-PE (Ptpn2376-384(RWLYWQPTL): H-2Kb) was provided by AtaGenix. To validate specificity, researchers stimulated BMDCs with seven neoantigenic peptides or wild-type peptides, then co-cultured them with spleen T cells from vaccinated mice. Ex vivo IFN-γ ELISPOT and flow cytometry showed significant T-cell activation in the NeoPol-mL242 group, with statistically increased frequencies of CD69+ and IFN-γ+ T cells, a 3.8-fold rise in spot-forming cells in a dose-dependent manner, and a 10.8% proportion of CD69+ NK cells, while wild-type controls showed no effect. Additionally, CD8+ T cells isolated from the vaccine group induced approximately 42% apoptosis in Hepa1-6 cells during co-culture, with significantly elevated TNF-α secretion (corresponding to Fig. 6L-N). These data confirm that NeoPol-mL242 induces neoantigen-specific T-cell immunity via DC-dependent pathways, enhancing tumor-killing capabilities.
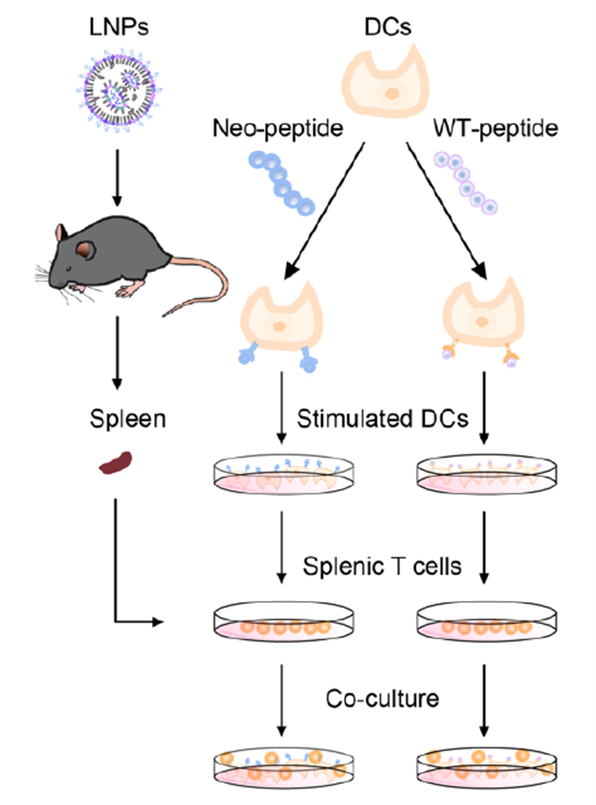
Figure 6. Schematic of BMDCs co-cultured with spleen T cells from immunized mice
Safety Evaluation of the NeoPol-mL242 Vaccine in Mice
Safety evaluation of the NeoPol-mL242 vaccine in mice involved serum biochemistry and histopathological analysis. Transient elevations in liver enzymes AST and ALT were observed, peaking at 24 hours and returning to normal within 72 hours, with no significant differences in ALP, ALB, or TP levels. ELISA quantification of serum inflammatory cytokines IL-1β, IL-6, TNF-α, and IP-10 showed transient increases 2 hours post-vaccination, returning to baseline within one week. Histopathology of major organs (liver, heart, spleen, lung, kidney) revealed no pathological changes, and no tissue damage was observed six months post-injection. Pharmacokinetic analysis of GFP mRNA-L242-20Lipo showed mRNA residual primarily in the spleen, nearly undetectable after 96 hours. These results confirm that NeoPol-mL242 has no acute toxicity, a favorable safety profile, and induces robust T-cell responses at low doses without significant inflammation or damage.
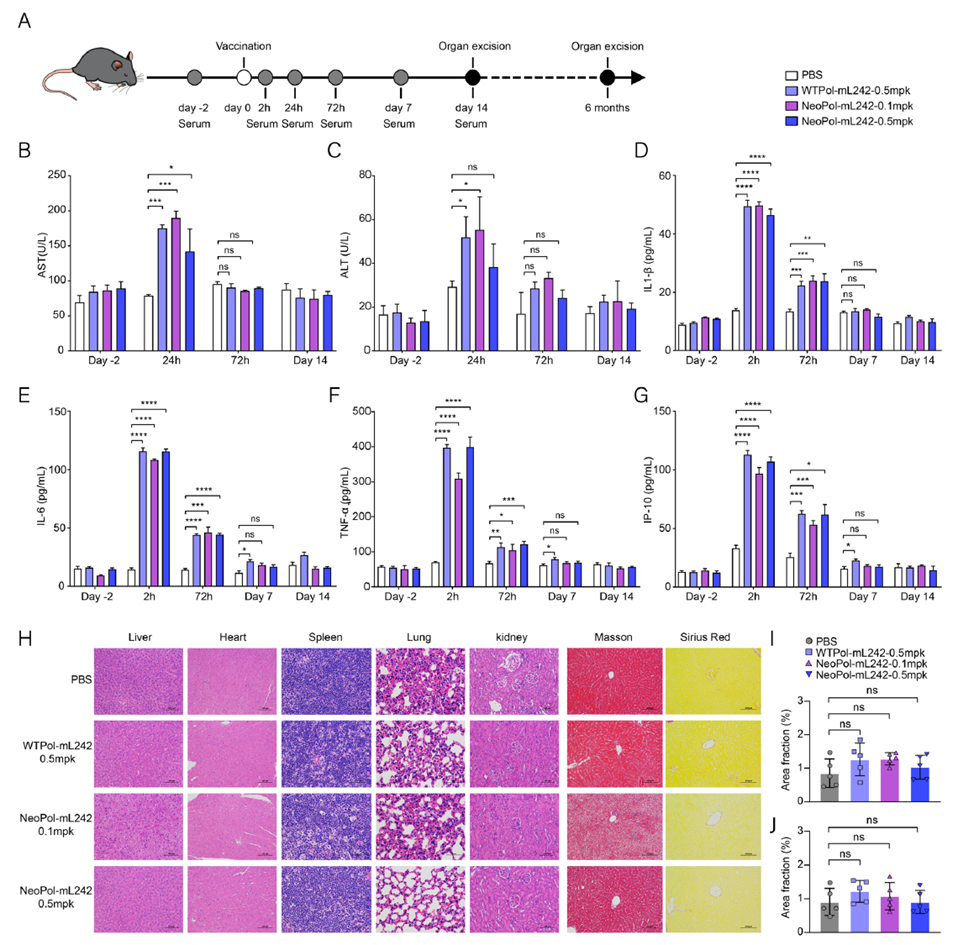
Figure 7. Safety evaluation of the NeoPol-mL242 mRNA vaccine in mice
This study focuses on the application of the spleen-targeting NeoPol-mL242 mRNA vaccine in a hepatocellular carcinoma (HCC) model. By optimizing the L242-20Lipo delivery system, efficient spleen-selective mRNA delivery and DC activation were achieved, significantly reducing hepatotoxicity. In melanoma and HCC models, the vaccine successfully induced robust T-cell responses, effectively inhibiting tumor growth and prolonging survival, demonstrating its outstanding potential in cancer immunotherapy.
Currently, mRNA vaccines like BNT111 have shown progress in melanoma, but liver-targeting limits their application in HCC. The innovative spleen-targeting LNPs, combined with AI prediction technology, promise to enhance efficacy and reduce side effects, signaling a new direction for future immunotherapies.
In this study, the key Anti-mouse MHC-Tetramer-PE (Ptpn2376-384 (RWLYWQPTL): H-2Kb) was custom-developed and provided by AtaGenix Laboratories Co., Ltd. (Wuhan, PR China). AtaGenix specializes in high-quality protein and antibody product development, offering one-stop services from antibody discovery and custom development to large-scale production, supporting researchers in advancing mechanistic studies and target validation.
As of September 2025, over 400 publications have cited AtaGenix’s one-stop protein and antibody development services. Looking ahead, AtaGenix will continue to support scientific innovation.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

