AtaGenix Laboratories
AtaGenix Laboratories
AtaGenix offers advanced mammalian protein expression services leveraging CHO and HEK293 cell systems. By integrating cutting-edge transient transfection platforms such as XtenCHO and FreeStyle CHO, we ensure near-native folding, proper glycosylation, and high-yield production of recombinant proteins. Our end-to-end workflow supports antigen preparation, antibody discovery, and biopharmaceutical development, providing research teams with consistent, scalable, and regulatory-ready protein solutions.
Professional and Efficient
10+ years of experience, 10,000+ antibodies/year, 300+ monoclonals.
High-Quality Assurance
ISO9001-certified, 100,000-grade cleanrooms, guaranteed purity.
Scalable Manufacturing Capability
2,000L+ annual capacity, from trials to large-scale production.
AtaGenix delivers a fully integrated CHO and HEK293 expression platform, combining proprietary culture media, advanced transfection reagents, and optimized purification workflows such as ion exchange chromatography and gel filtration. This system ensures high-yield recombinant protein and antibody production with two major advantages:
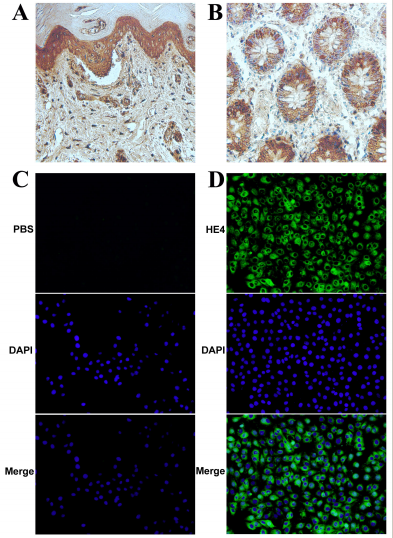
A 2017 study developed a high-affinity anti-HE4 monoclonal antibody (9C3) using AtaGenix’s advanced mammalian expression and hybridoma platforms. This breakthrough enhances ovarian cancer diagnostics by targeting the HE4 biomarker with high specificity, offering potential for early detection and therapy.
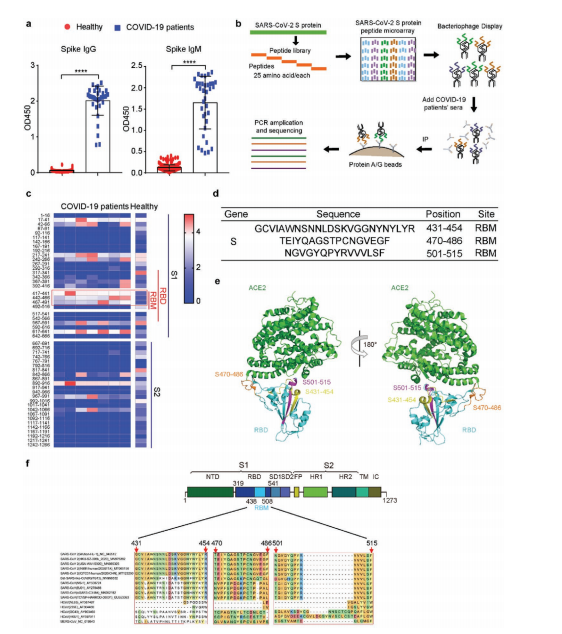
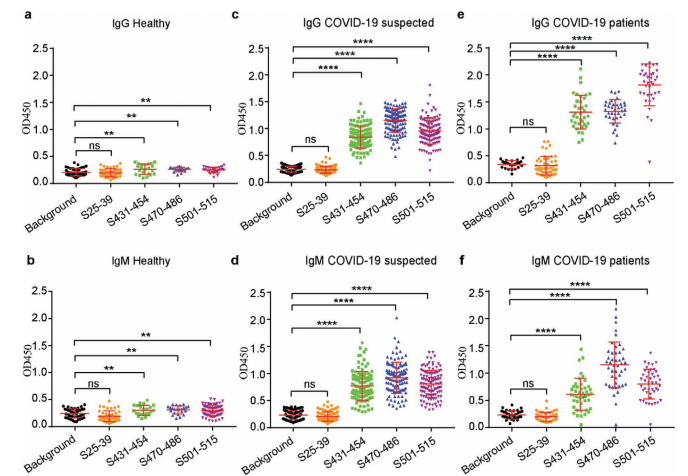
This article highlights a 2021 Small Methods study that identified immunogenic peptides in the SARS-CoV-2 spike protein and isolated neutralizing monoclonal antibodies from COVID-19 patients. The research pinpointed key RBM epitopes (S431-454, S470-486, S501-515), with S470-486 emerging as an immunodominant target for diagnostics and therapeutics. Using phage display and ScFv libraries, the study validated epitope mapping and antibody functionality. Supported by AtaGenix’s expertise in phage display, protein expression, and assay development, this breakthrough offers a scalable approach for combating emerging viral pathogens, updated as of August 24, 2025.