AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-10-17 View volume: 655
Gastric cancer (GC) is the fifth most common malignant tumor globally and the fourth leading cause of cancer-related deaths. A major challenge in its treatment is chemotherapy resistance, particularly to oxaliplatin (OXA). As a first-line chemotherapy drug for GC, OXA resistance involves complex mechanisms, including epigenetic dysregulation and metabolic reprogramming, such as enhanced glycolysis and excessive lactate production driven by the Warburg effect. In recent years, lactate has been redefined as a key epigenetic modifier, influencing pathological processes in infections and cancers through histone and non-histone lactylation. However, the role of lactylation in GC, especially in OXA resistance, remains poorly understood. Meanwhile, long non-coding RNAs (LncRNAs), as critical epigenetic regulators, influence GC resistance by modulating energy metabolism, but the specific mechanisms are unclear.
A research team from Jiangnan University Affiliated Hospital, published in Free Radical Biology and Medicine, integrated multi-omics analysis and organoid models to discover that LncRNA BASP1-AS1 drives lactylation at the K115 site of Poly(RC)-binding protein 2 (PCBP2K115la), suppressing ferroptosis and conferring OXA resistance in GC cells. Mechanistically, BASP1-AS1 recruits the Unc-51 Like Autophagy Activating Kinase 1 (ULK1) and lactate dehydrogenase A (LDHA) complex, promoting LDHA phosphorylation to enhance glycolysis and lactate production. Lactate-induced PCBP2K115la blocks its interaction with ARIH2, inhibiting ubiquitination-mediated degradation and stabilizing PCBP2, while also transcriptionally activating LDHA/PCBP2 via histone H3K14la, forming a self-amplifying metabolic-epigenetic loop. This axis suppresses ferroptosis to maintain resistance, offering a novel target for overcoming GC OXA resistance.
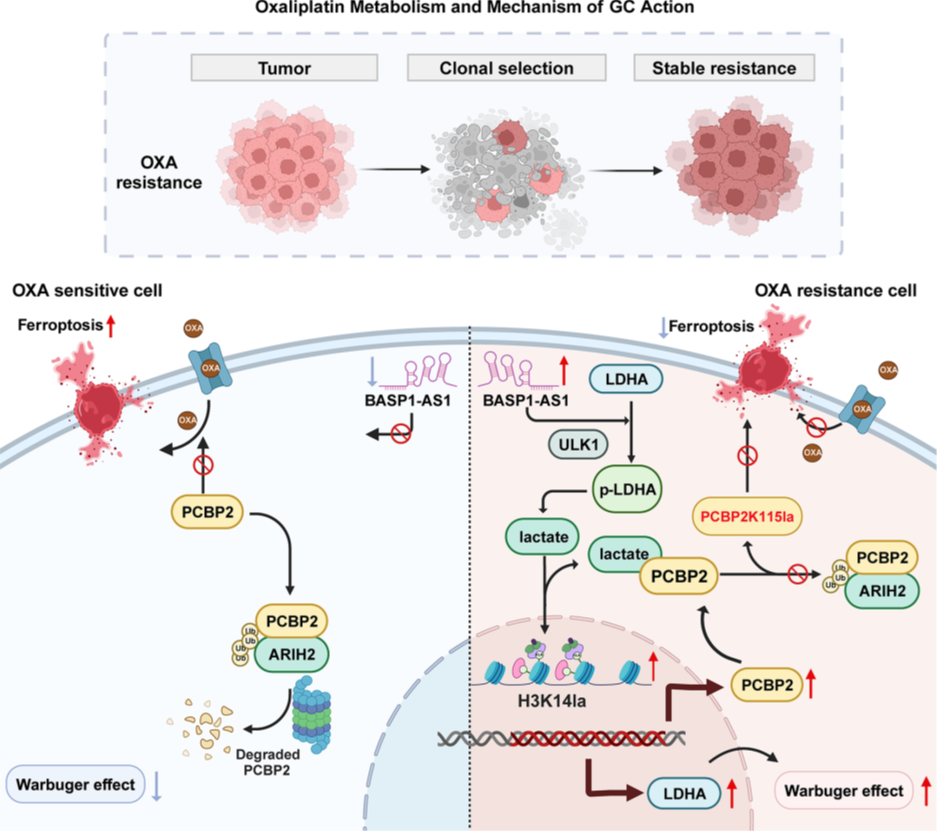
Figure 1. Signal pathway diagram
Lactate-induced PCBP2 upregulation confers OXA resistance in GC cells
To elucidate the mechanisms of OXA resistance in GC, the researchers established OXA-resistant cell lines (AGSR, MKN45R) and performed multi-omics analysis compared to sensitive parental lines (AGSWT, MKN45WT). CCK-8 assays confirmed significantly higher IC50 values in resistant cells. Transcriptomic and proteomic analyses revealed enrichment of anaerobic glycolysis and ferroptosis-related pathways in resistant cells, suggesting that metabolic reprogramming suppresses ferroptosis. Measurements of lactate production and extracellular acidification rate confirmed elevated glycolytic activity in resistant cells. Western blot (WB) analysis showed overall upregulation of protein lactylation in resistant cells, and pan-protein lactylation sequencing combined with differential expression and ferroptosis-related protein analysis identified PCBP2 as a key candidate: it was overexpressed at both transcriptional and protein levels in resistant cells and regulated by lactylation. WB confirmed PCBP2 upregulation in resistant cells, and KEGG analysis showed enrichment of lactate synthesis pathways. Exogenous lactate dose-dependently induced PCBP2 upregulation. Lentiviral-mediated PCBP2 knockdown and overexpression, validated by WB, showed that knockdown reduced OXA resistance (enhancing chemotherapy sensitivity), while overexpression increased resistance. Apoptosis assays indicated that knockdown increased cell death, but overexpression had no significant effect. These results establish PCBP2 as a critical regulator of OXA resistance, with its inhibition restoring sensitivity by inducing apoptosis.
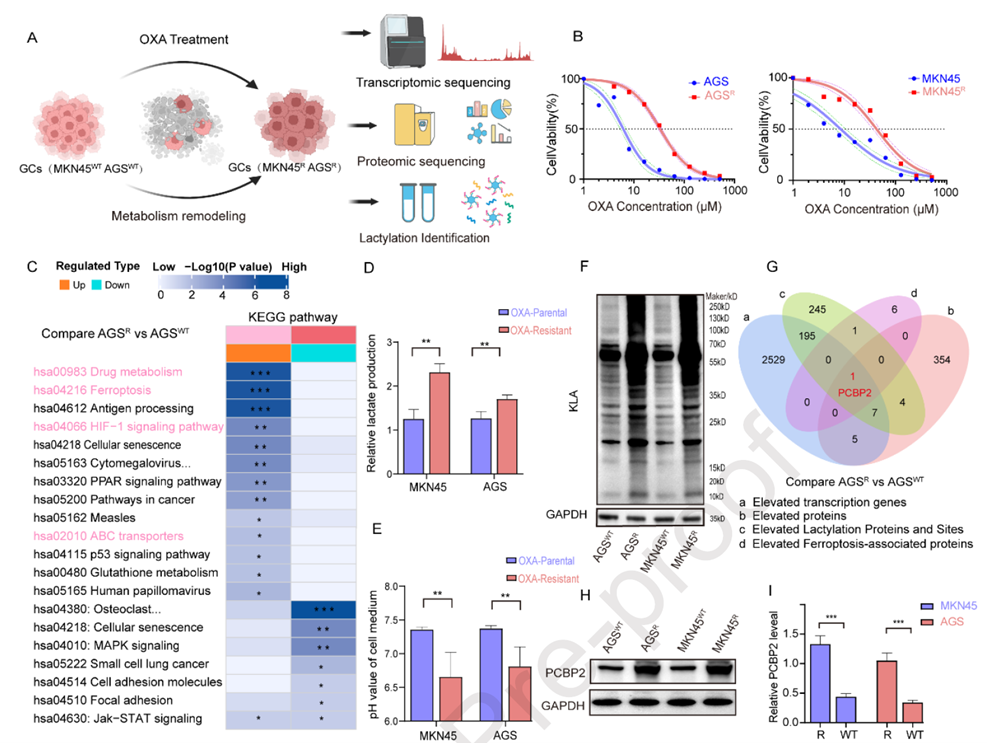
Figure 2. Lactate-induced PCBP2 upregulation leads to OXA resistance in GC cells
PCBP2K115 lactylation confers OXA resistance by blocking ARIH2-mediated ubiquitination
To investigate the cause of PCBP2 upregulation in resistant cells, the researchers conducted lactylation proteomics, revealing that many differentially lactylated proteins were enriched in ferroptosis pathways. Further screening identified lactylation at the critical iron-binding site K115 of PCBP2, as well as at K115 of PCBP1 and K45 of GCLM. Subsequent immunoprecipitation (IP) and WB experiments showed significantly reduced ubiquitination and enhanced K115 lactylation of PCBP2 in resistant cells. To explore their relationship, the researchers mutated K115 to arginine (K115R), which blocked lactylation and promoted PCBP2 ubiquitination, suggesting mutually exclusive regulation between lactylation and ubiquitination at this site. To identify the responsible E3 ligase, the team used Co-IP and mass spectrometry to map PCBP2’s E3 network, pinpointing ARIH2 as a key candidate. Structural modeling and binding experiments confirmed direct interaction between PCBP2 and ARIH2. In resistant cells, their binding was tighter, while ARIH2 knockdown reduced PCBP2 ubiquitination. Notably, the K115R mutation enhanced PCBP2-ARIH2 binding and decoupled regulation from lactate metabolism. These findings position K115 as a conformational switch: lactylation sterically hinders ARIH2-mediated ubiquitination, stabilizing PCBP2 and promoting OXA resistance, revealing metabolic-epigenetic crosstalk.
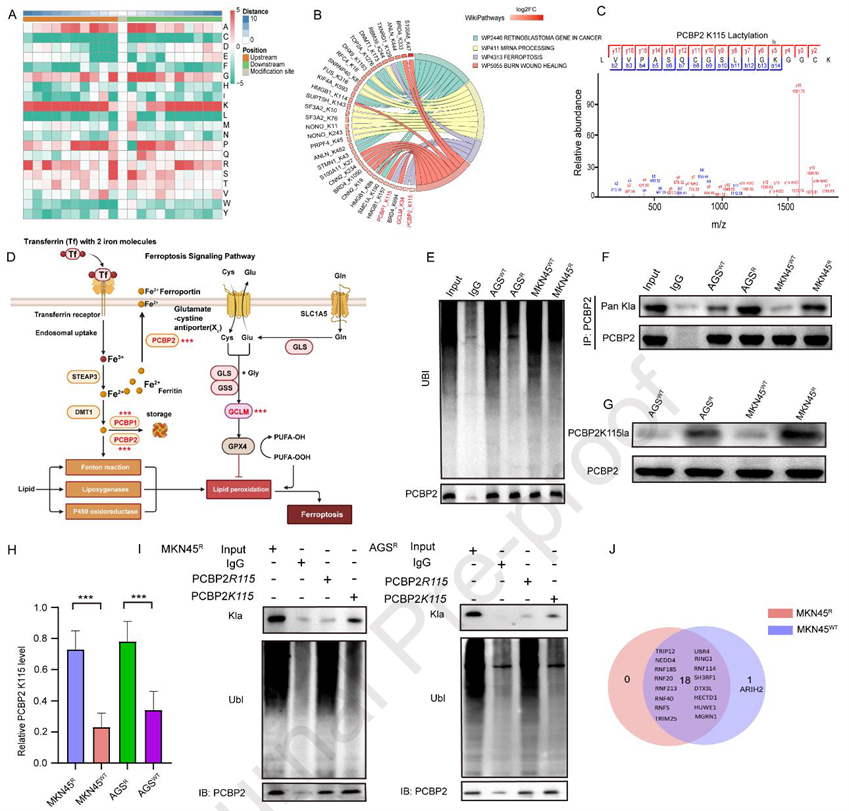
Figure 3. PCBP2K115 lactylation confers OXA resistance by blocking ARIH2-mediated ubiquitination
PCBP2 confers OXA resistance in GC patient-derived organoid models
To validate PCBP2’s role in clinical settings, the researchers established GC patient-derived organoids (PDOs), with H&E staining confirming retention of original tumor structures. Long-term monitoring revealed growth differences among PDOs under OXA treatment, and CCK-8 assays showed variable OXA responsiveness among patients, consistent with clinical outcomes. Protein analysis indicated that PCBP2 expression positively correlated with resistance indices, with higher levels in resistant PDOs. Lactate stimulation dose-dependently upregulated PCBP2 in PDOs from patients #2 and #3, while lentiviral PCBP2 knockdown reduced, and overexpression enhanced, OXA resistance. Additionally, the glycolysis inhibitor 2-DG attenuated the resistant phenotype. These findings suggest that PCBP2 is a potential predictive biomarker for OXA response, significantly influenced by lactate metabolism.
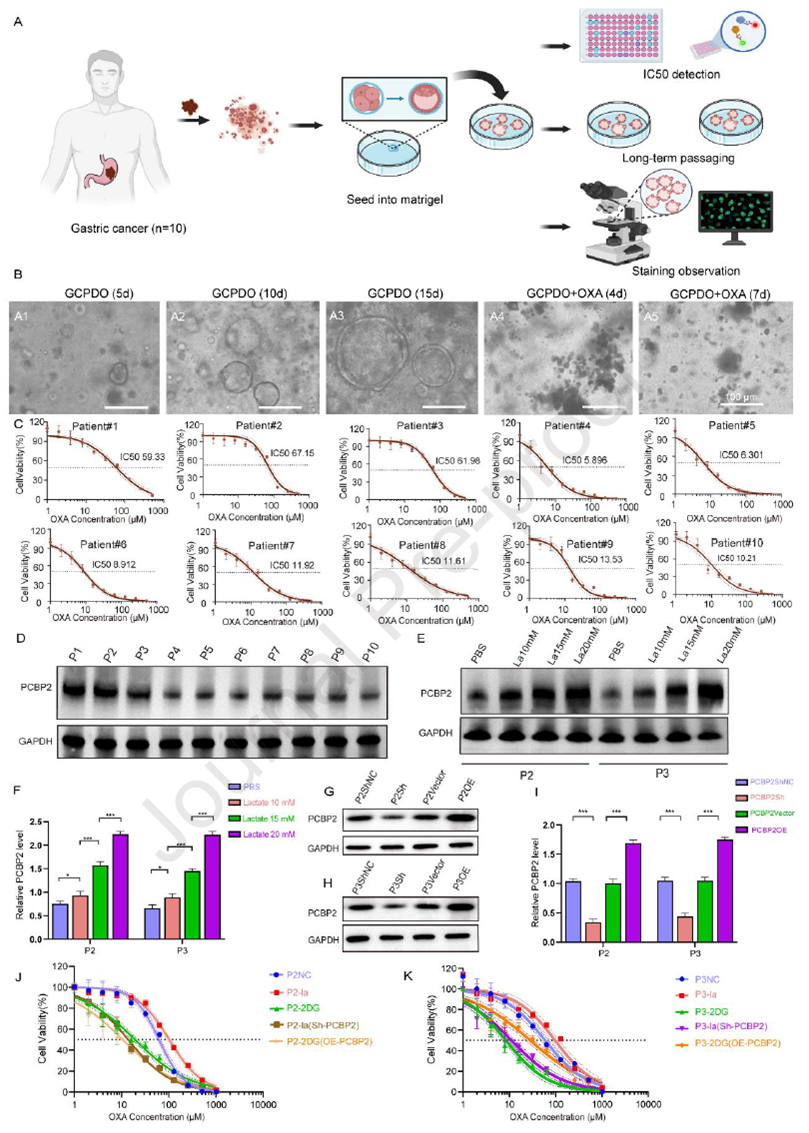
Figure 4. PCBP2 confers OXA resistance in GC patient-derived organoid models
BASP1-AS1 suppresses ferroptosis and upregulates PCBP2 expression
The researchers found that ferroptosis is closely linked to OXA sensitivity: the ferroptosis inhibitor Fer-1 increased OXA IC50, while the inducer RSL3 reduced it in resistant cells. Given PCBP2’s role in ferroptosis regulation, the study focused on its upstream LncRNAs. TCGA-STAD and transcriptomic analyses identified four candidate LncRNAs, with BASP1-AS1 highly expressed in GC tissues and associated with poor prognosis. qRT-PCR and FISH confirmed that BASP1-AS1 was elevated in resistant cells and high-grade tissues, co-localizing with PCBP2. Functional experiments showed that BASP1-AS1 knockdown reduced PCBP2 expression, increased ROS accumulation and ferroptosis, inhibited proliferation, and enhanced OXA sensitivity, while overexpression had the opposite effect. These results reveal that BASP1-AS1, as an upstream regulator of PCBP2, maintains OXA resistance and tumor growth by suppressing ferroptosis and apoptosis.
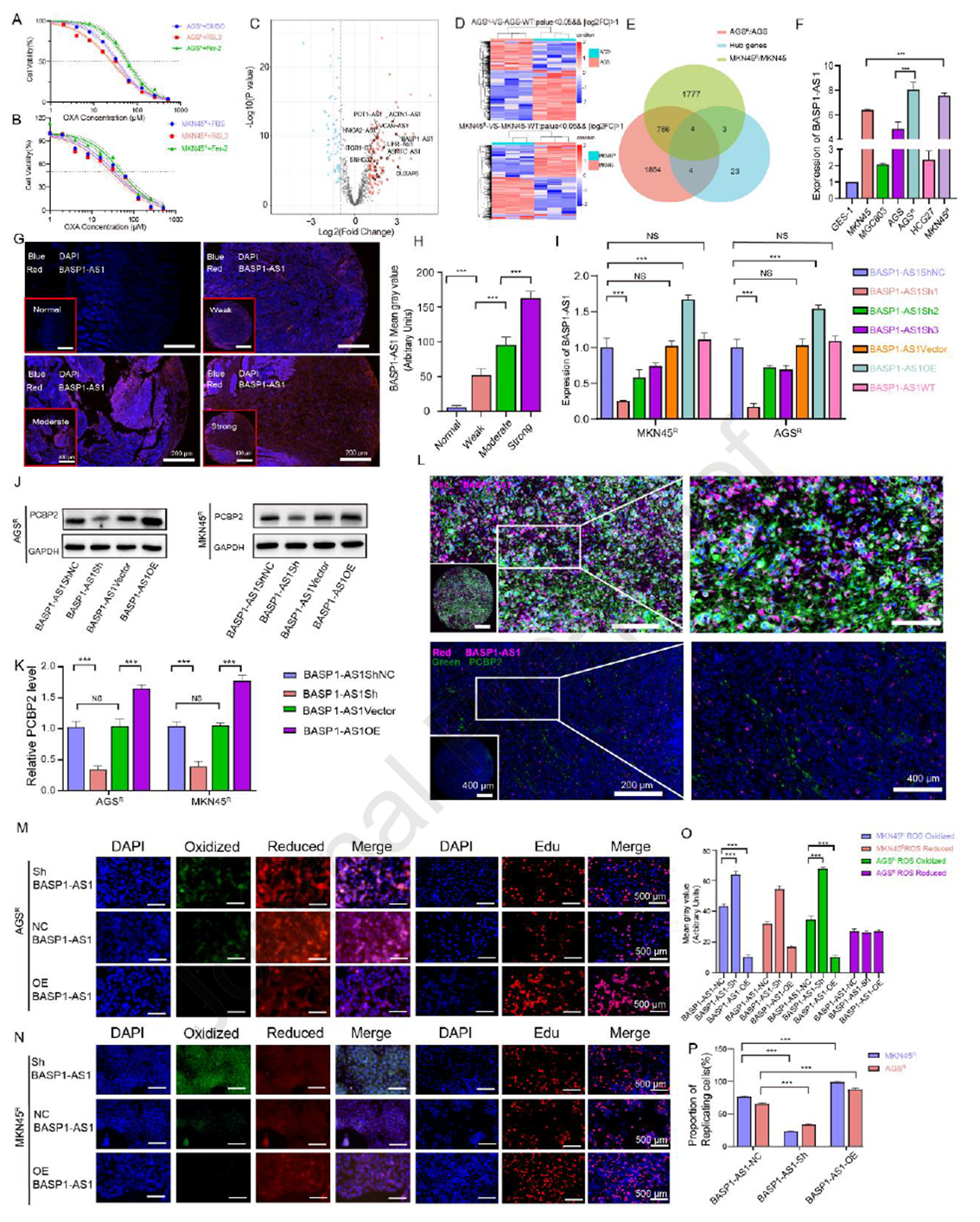
Figure 5. BASP1-AS1 suppresses ferroptosis and upregulates PCBP2 expression
BASP1-AS1 activates LDHA-dependent lactate accumulation to enhance PCBP2K115la-mediated ubiquitination suppression
Further investigation into BASP1-AS1’s regulation of PCBP2 revealed that BASP1-AS1 not only affects gene expression but also reshapes cellular metabolism. It drives resistant cells toward anaerobic glycolysis, increasing lactate levels and reinforcing resistance through an acidic microenvironment. Inhibiting glycolysis weakened this advantage. In-depth analysis showed that BASP1-AS1 promotes PCBP2 lactylation, preventing its degradation and stabilizing its accumulation, while blocking ARIH2-mediated ubiquitination. Tracing upstream mechanisms, the team found that BASP1-AS1 activates ULK1 and engages LDHA, accelerating the glycolytic pathway. Ultimately, metabolism and epigenetic modifications form a closed loop: BASP1-AS1 fuels glycolysis, creates a lactate-rich environment, and protects PCBP2, driving resistance.
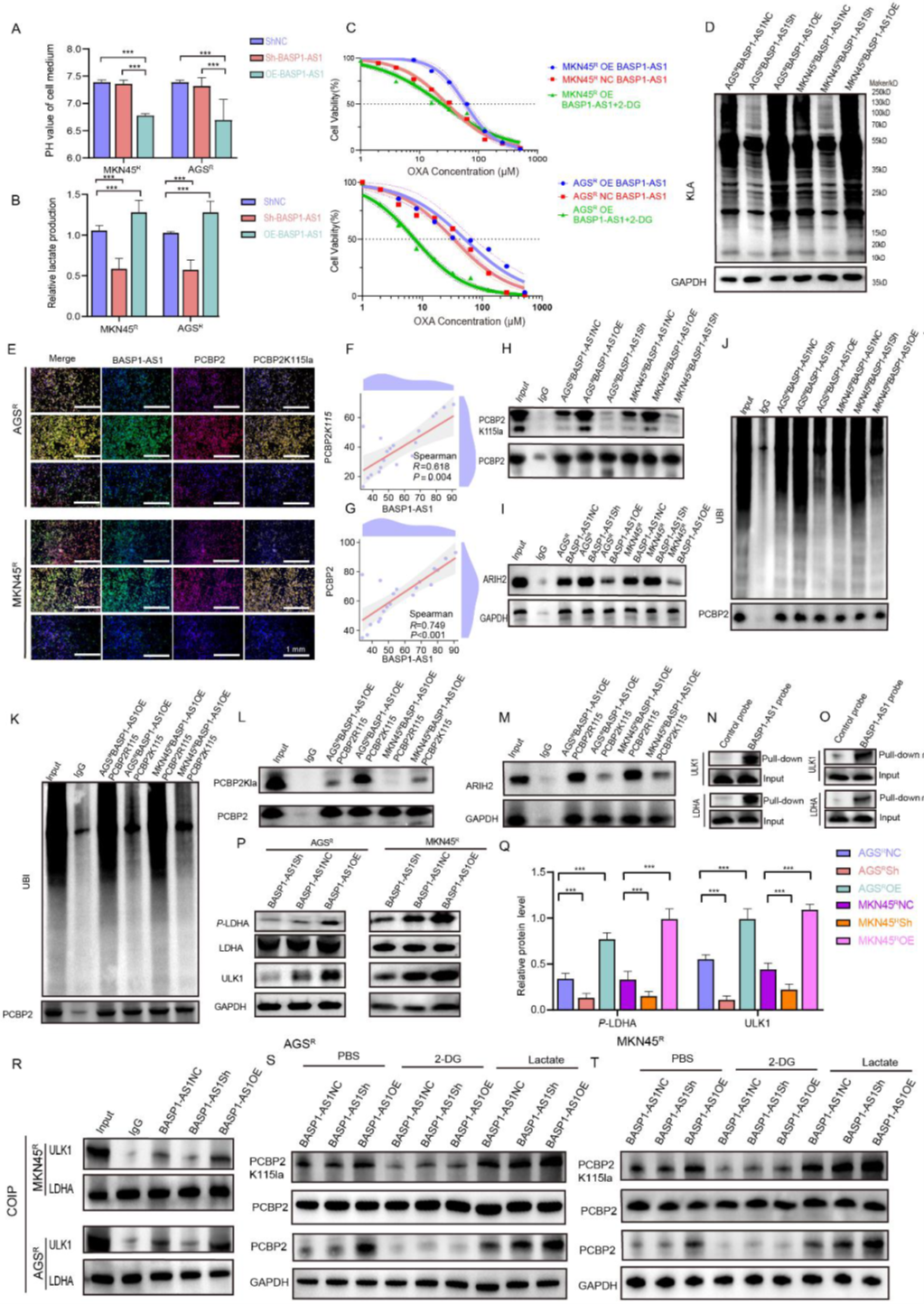
Figure 6. BASP1-AS1 activates LDHA-dependent lactate accumulation to enhance PCBP2K115la-mediated ubiquitination suppression
BASP1-AS1 suppresses ferroptosis in GC cells via the PCBP2 pathway
In rescue experiments, the researchers confirmed whether BASP1-AS1’s regulation of OXA sensitivity and ferroptosis depends on PCBP2. Results showed that even with BASP1-AS1 knockdown, PCBP2 overexpression increased ROS levels but could not reverse the reduced resistance, suppressed proliferation, and increased apoptosis caused by knockdown. Electron microscopy further revealed denser, more active mitochondrial structures in BASP1-AS1-knockdown cells, with PCBP2’s compensatory effect remaining limited. Collectively, BASP1-AS1 suppresses ferroptosis through PCBP2-related but not entirely dependent pathways, maintaining resistance, suggesting it as a potential target for overcoming OXA resistance.
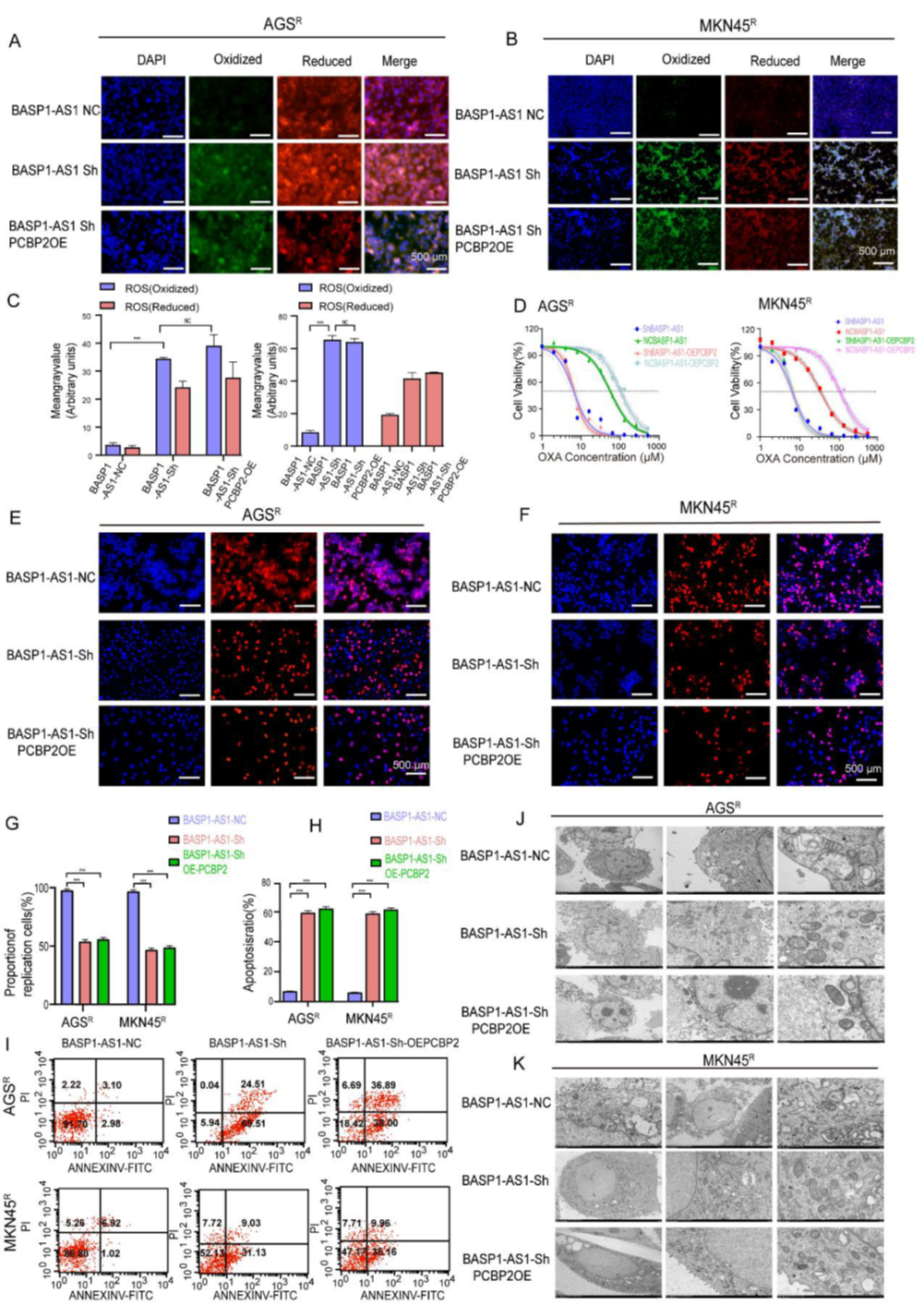
Figure 7. BASP1-AS1 suppresses ferroptosis in GC cells via the PCBP2 pathway
BASP1-AS1 activates H3K14la and LDHA-dependent PCBP2K115la to establish a positive feedback loop
The researchers found that BASP1-AS1 knockdown significantly altered the protein lactylation landscape, particularly histone H3K14 lactylation. Further experiments showed that this modification depends on glycolysis-derived lactate: inhibiting glycolysis reduced H3K14 lactylation, while exogenous lactate reversed this decline. Cut&Tag analysis revealed that BASP1-AS1 altered H3 binding patterns, strongly activating promoter regions of ferroptosis- and glycolysis-related genes (e.g., LDHA and PCBP2). Using AtaGenix-customized anti-PCBP2K115la rabbit polyclonal antibodies to detect key modification sites, the researchers confirmed that BASP1-AS1 enhances glycolysis to promote LDHA and PCBP2 transcription, forming a “metabolic-epigenetic” positive feedback loop that further accumulates PCBP2 lactylation, suppresses ferroptosis, and enhances OXA resistance. In vivo experiments showed that BASP1-AS1-overexpressed mouse tumors had reduced OXA response, while combined PCBP2 knockdown restored drug sensitivity, validating key nodes in this mechanism.
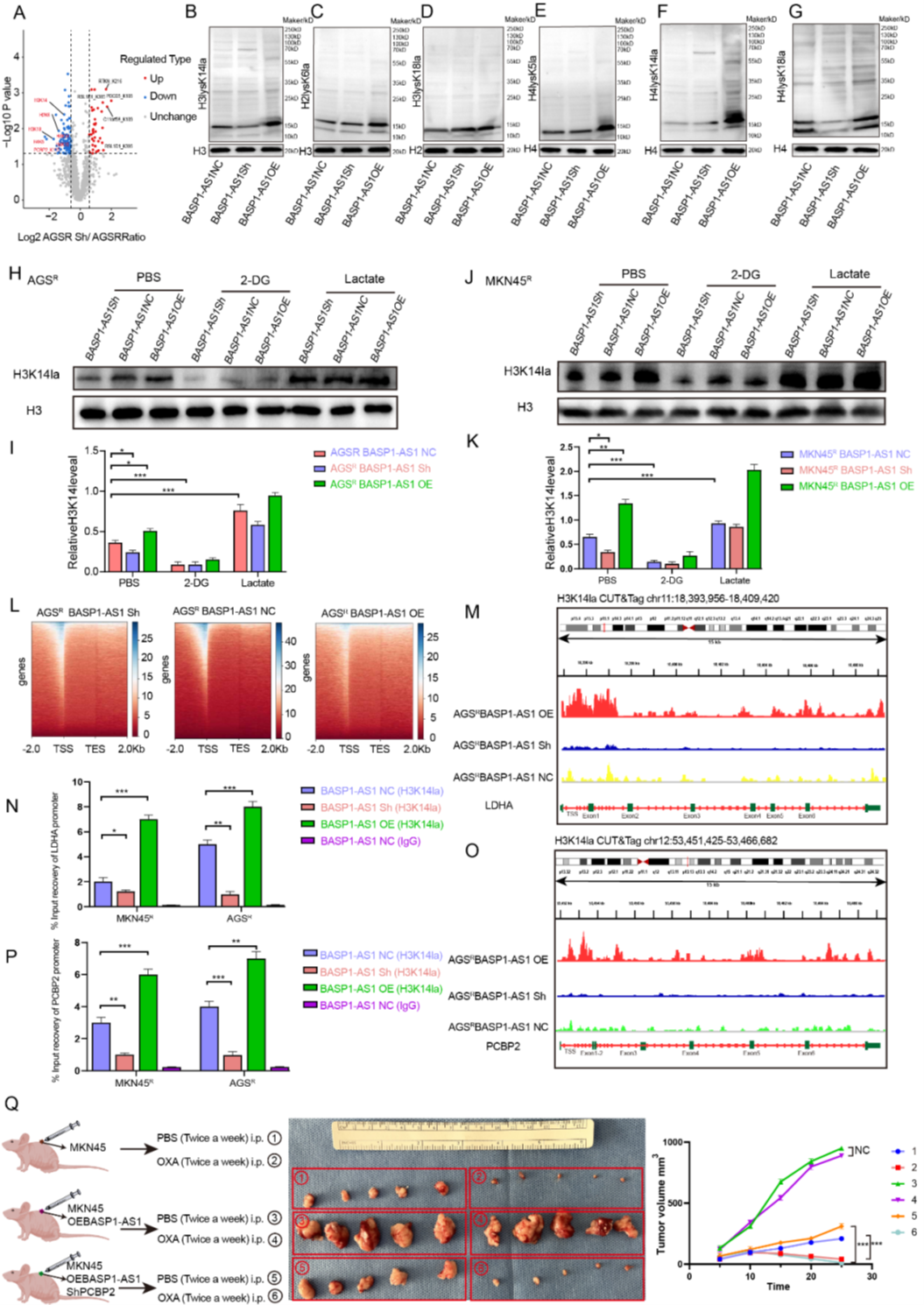
Figure 8. BASP1-AS1 activates H3K14la and LDHA-dependent PCBP2K115la to establish a positive feedback loop
In summary, this study reveals that in OXA-resistant GC cells, the BASP1-AS1/ULK1/LDHA axis promotes lactate production, inducing PCBP2K115la to block ARIH2-mediated ubiquitination, stabilizing PCBP2, and transcriptionally activating LDHA/PCBP2 via H3K14la to form a self-amplifying loop. This axis suppresses ferroptosis to maintain resistance, highlighting the BASP1-AS1–PCBP2 pathway as a key molecular mechanism and potential therapeutic target for GC OXA resistance. The high specificity of AtaGenix-provided anti-PCBP2K115la antibodies enabled precise tracking of lactylation dynamics, clarifying the molecular basis of BASP1-AS1’s regulation of PCBP2 stability and OXA resistance, providing robust technical support for studying metabolic-epigenetic interactions.
As of October 2025, over 400 publications have cited AtaGenix’s one-stop protein and antibody development services. Looking ahead, AtaGenix will continue to fully support scientific research, driving technological innovation and breakthroughs.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

