AtaGenix Laboratories
AtaGenix Laboratories
AtaGenix’s naïve library technology enables rapid antibody discovery with high diversity (up to 10¹⁰ variants) while eliminating the need for animal immunization. Compared to hybridoma technology, our phage display approach reduces turnaround time to 3-4 months and removes the need for further humanization, ensuring efficient and cost-effective therapeutic antibody development.
Rapid Antibody Screening
Select high-affinity antibodies within 3-4 months, twice as fast
Animal-Free & Ethical
No animal immunization, fully human naïve library screening
No Further Humanization Needed
Direct selection of fully human antibodies, reducing costs
| Library | Format | Species | Size(Capacity) |
|---|---|---|---|
| LiAb-SFCOVID-19™ | scFv | Human – COVID-19 recovered donors | 1.35 × 10¹⁰ |
| LiAb-SFMAX™ | scFv | Human – 5 ethnic groups, 368 donors | 7.38 × 10¹⁰ |
| LiAb-SFAutoimmune™ | scFv | Human – with autoimmune disease | 4.78 × 10⁹ |
| LiAb-VHHMAX™ | VHH | Camel, llama, alpaca , 76 donors | 2.71 × 10¹⁰ |
| LiAb-SFDab™ | scFv | Dog – 6 breeds,26 donors | 1.52 × 10¹⁰ |
| LiAb-SFCab™ | scFv | Cat – 10 breeds,78 donors | 1.67 × 10¹⁰ |
| LiAb-SFRab™ | scFv | Rabbit– 5 breeds,64 donors | 2.95 × 10¹⁰ |
| LiPep-12 | Peptide 12-mer | / | 1.00 × 10⁹ |
| LiPep-7 | Peptide 7-mer | / | 1.00 × 10⁹ |
Example of the panning (biopanning) protocol in Phage Display.
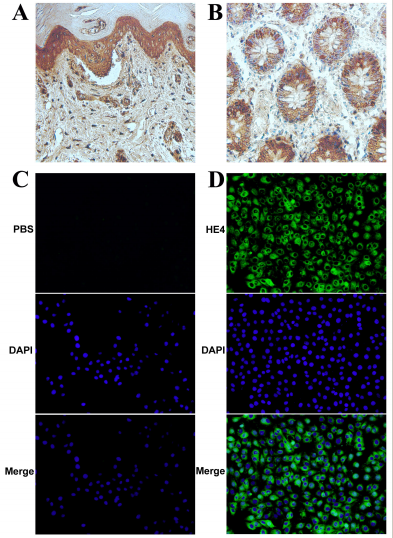
A 2017 study developed a high-affinity anti-HE4 monoclonal antibody (9C3) using AtaGenix’s advanced mammalian expression and hybridoma platforms. This breakthrough enhances ovarian cancer diagnostics by targeting the HE4 biomarker with high specificity, offering potential for early detection and therapy.
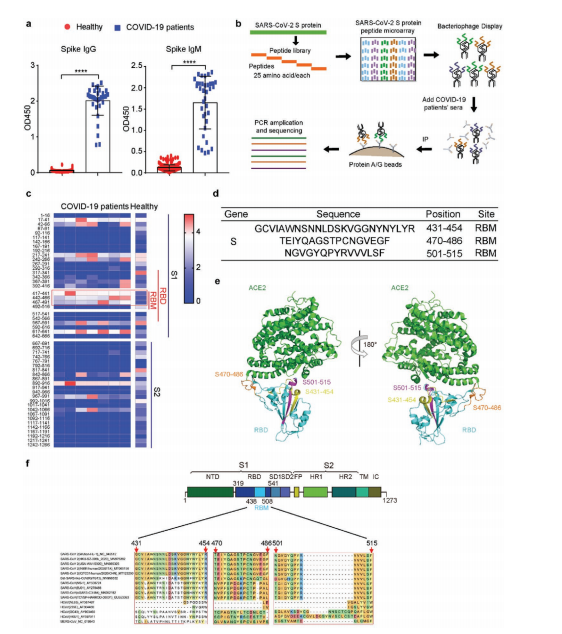
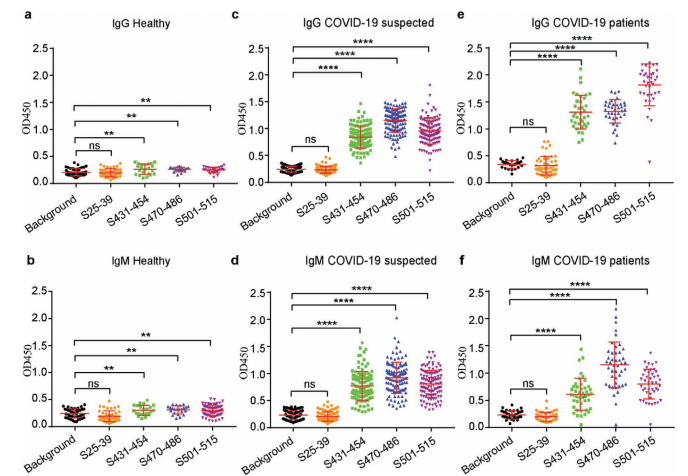
This article highlights a 2021 Small Methods study that identified immunogenic peptides in the SARS-CoV-2 spike protein and isolated neutralizing monoclonal antibodies from COVID-19 patients. The research pinpointed key RBM epitopes (S431-454, S470-486, S501-515), with S470-486 emerging as an immunodominant target for diagnostics and therapeutics. Using phage display and ScFv libraries, the study validated epitope mapping and antibody functionality. Supported by AtaGenix’s expertise in phage display, protein expression, and assay development, this breakthrough offers a scalable approach for combating emerging viral pathogens, updated as of August 24, 2025.