The Human Major Histocompatibility Complex (MHC), also known as Human Leukocyte Antigen (HLA), plays a pivotal role in the immune system by distinguishing self from non-self molecules. Located on chromosome 6, MHC genes encode proteins that present antigens to T cells, initiating adaptive immune responses. MHC molecules are categorized into three classes—MHC I, MHC II, and MHC III—each with specialized functions in immunity.
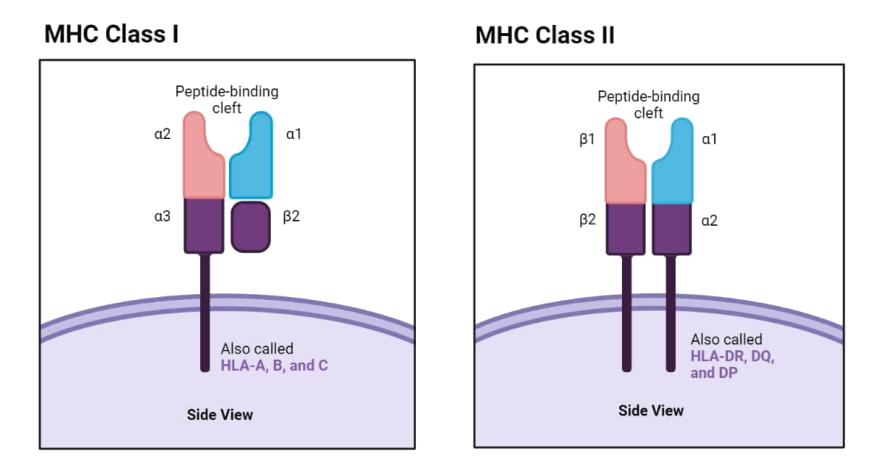
Figure 1. Structure of MHC I Molecule
MHC I: Presenting Intracellular Antigens
MHC I molecules are expressed on nearly all nucleated cells and consist of an alpha chain paired with β2-microglobulin (B2M). The alpha chain includes three domains (α1, α2, α3), with α1 and α2 forming a peptide-binding groove that holds peptides (8–10 amino acids) from intracellular sources, such as viral proteins or tumor antigens. These MHC I–peptide complexes are recognized by the T-cell receptor (TCR) on CD8+ cytotoxic T cells, triggering targeted cell destruction. The TCR’s affinity for monomeric MHC I–peptide complexes is modest but is significantly enhanced with MHC I tetramers, improving detection of antigen-specific T cells.
MHC I is essential for antiviral immunity, tumor surveillance, and transplant compatibility. Dysregulation of MHC I presentation can lead to immune evasion in cancers or contribute to autoimmune disease.
MHC II: Orchestrating Adaptive Immunity
MHC II molecules are primarily found on antigen-presenting cells (APCs) like dendritic cells, macrophages, and B cells. Composed of α and β chains, the α1 and β1 domains form the peptide-binding groove, which binds extracellular antigens (13–25 amino acids), such as bacterial peptides. MHC II presents these antigens to CD4+ T helper cells, which coordinate immune responses by activating B cells for antibody production and macrophages for pathogen clearance.
MHC II is crucial for immunity against extracellular pathogens and is implicated in multiple autoimmune diseases due to genetic polymorphisms and altered antigen presentation.
MHC III: Supporting Immune Regulation
MHC III genes encode immune-supporting proteins rather than antigen-presenting molecules. These include complement components (C2, C4, factor B), cytokines (e.g., TNF-α), and heat shock proteins. Positioned between MHC I and MHC II regions, MHC III proteins enhance innate immunity, inflammation, and immune signaling, complementing the adaptive roles of MHC I and MHC II.
Polymorphisms in MHC III genes are linked to inflammatory disorders, making them key targets for immunomodulation research.
Custom MHC I Complex Services at AtaGenix
AtaGenix offers custom MHC I complex services to support advanced immunological research. Leveraging our eukaryotic expression platform (HEK293), we ensure native-like folding and glycosylation for MHC I complexes, avoiding misfolding often seen in prokaryotic systems. Our process includes:
We validate MHC complexes using techniques such as SPR and FACS to ensure high biological activity, supporting applications in T-cell analysis, vaccine evaluation, and drug discovery.
Applications in Research and Therapeutics
MHC molecules underpin both research and clinical practice. HLA typing is vital in transplantation to reduce rejection by matching donor–recipient HLA profiles. In vaccine design, peptide–MHC binding insights guide epitopes that elicit robust T-cell responses. MHC tetramers quantify antigen-specific T cells, accelerating immunotherapy programs in cancer and infectious disease.
Therapeutically, modulating MHC pathways can address autoimmunity and tumor immune evasion. Custom MHC I complexes are essential for TCR-T cell therapy discovery and TCR-like antibody development, enabling precision immuno-oncology.
About AtaGenix
AtaGenix supplies high-quality bioreagents and custom CRO services for MHC research. Representative offerings include:
Talk to our PhD team about peptide selection, alleles, and QC options.