AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-08-28 View volume: 672
In this recent study published in Immunity, the research team employed a high-purity, custom-produced MOG protein supplied by AtaGenix, which enabled them to successfully establish an experimental autoimmune encephalomyelitis (EAE) model and demonstrate the pivotal role of meningeal B cells in multiple sclerosis (MS) relapse. As the core antigen, the MOG protein not only ensured experimental stability and reproducibility but also provided critical support in uncovering the pathogenic interactions between B cells and T cells.
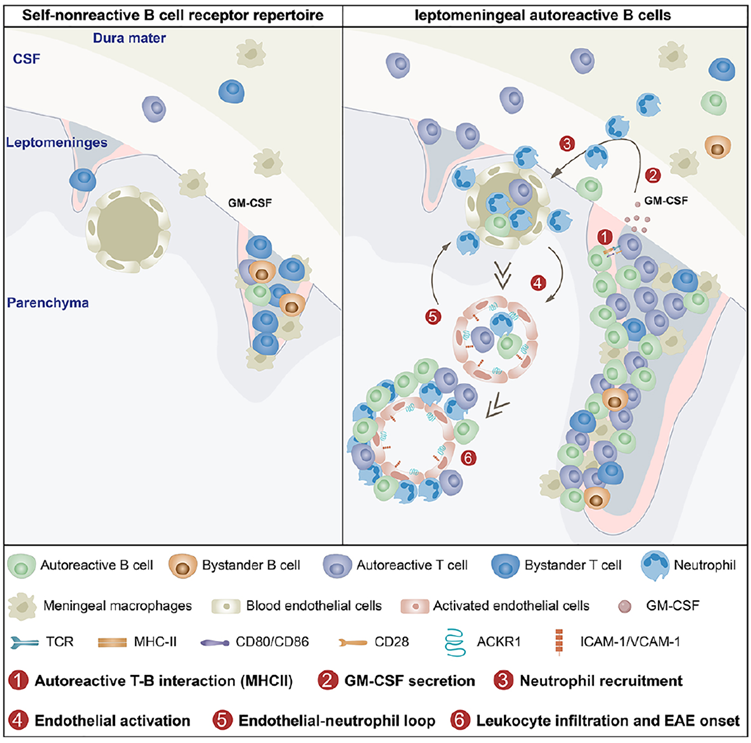
Figure 1. Graphical abstract
Under normal conditions, meningeal B cells maintain strict immune tolerance. However, when autoreactive B cells targeting CNS antigens were introduced, they became specifically activated within the leptomeninges and acquired antigen-presenting capacity. This activation depended on the presence of CNS autoantigens. The study confirmed that myelin oligodendrocyte glycoprotein (MOG) is the key antigen, as mice lacking MOG failed to trigger B-cell activation, underscoring MOG’s central role in driving this pathological process. Importantly, the high-purity, biologically active MOG protein used in these experiments was provided by AtaGenix, ensuring model consistency and reliable data.
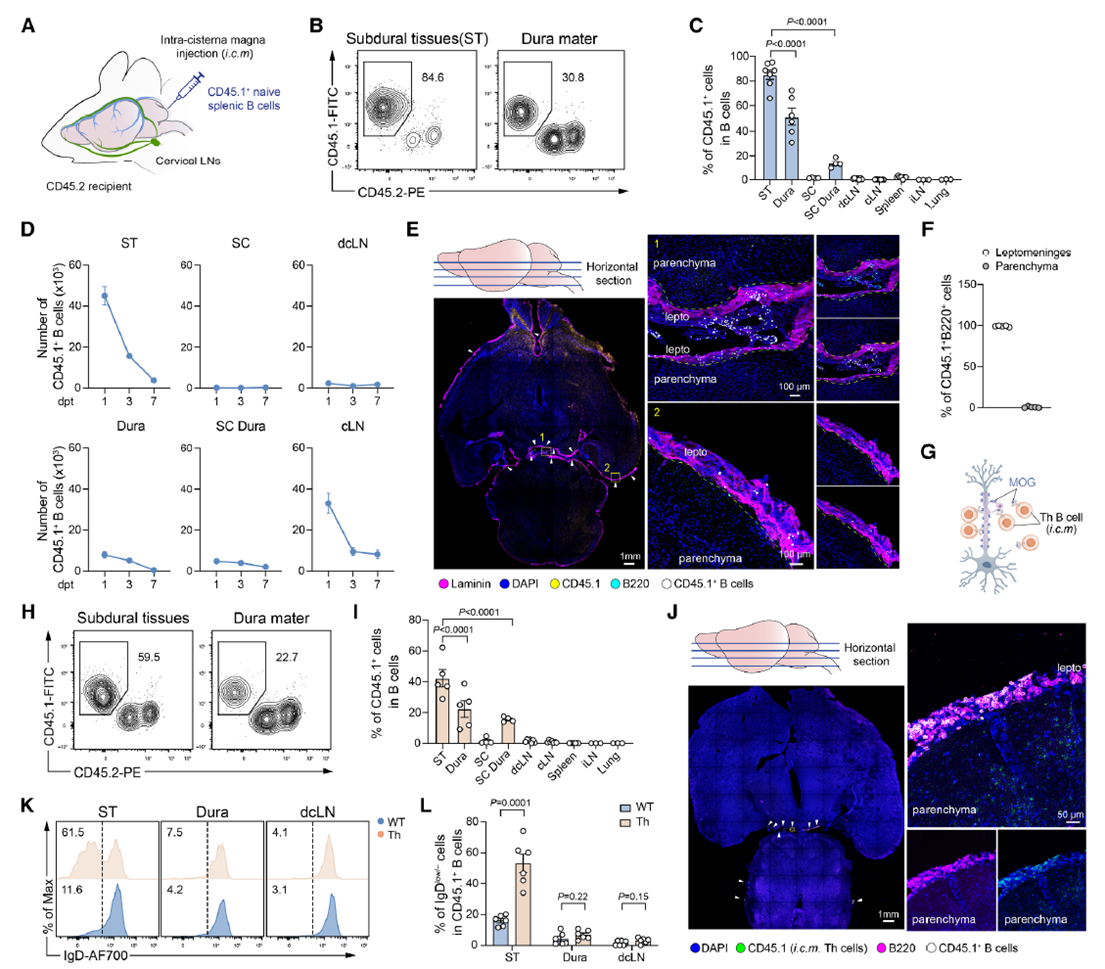
Figure 2. Meningeal autoreactive B cells promote T cell-mediated CNS inflammation through antigen presentation
The researchers further showed that autoreactive meningeal B cells present MOG antigens to pathogenic T cells via MHC-II molecules, leading to rapid T-cell activation and robust GM-CSF production. This cytokine in turn recruited neutrophils into the meninges, amplifying the inflammatory response. Once present, neutrophils activated vascular endothelial cells, inducing the expression of adhesion molecules such as VCAM-1 and ACKR1, which facilitated further immune-cell infiltration. The result was a self-sustaining inflammatory loop involving B cells, T cells, neutrophils, and endothelial cells, driving disease progression.
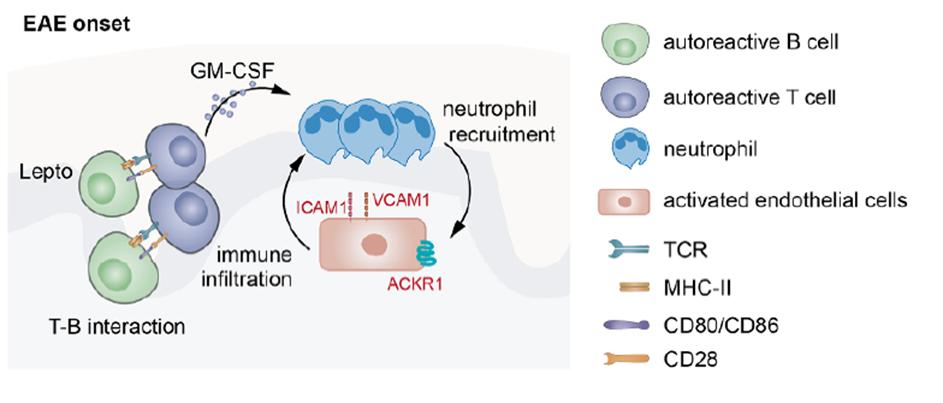
Figure 3. A proinflammatory loop between GM-CSF-dependent neutrophil recruitment and endothelial cell activation
In a relapsing–remitting EAE model, local intracisternal injection of low-dose anti-CD20 antibodies selectively depleted brain-resident B cells without affecting peripheral B cells. This intervention significantly reduced relapse severity, suppressed T-cell proliferation, lowered pro-inflammatory cytokine release, and diminished endothelial activation. Single-cell sequencing further revealed substantial clonal expansion of brain B cells during relapse, with persistence even into remission, highlighting their role as both initiators and drivers of recurrence.
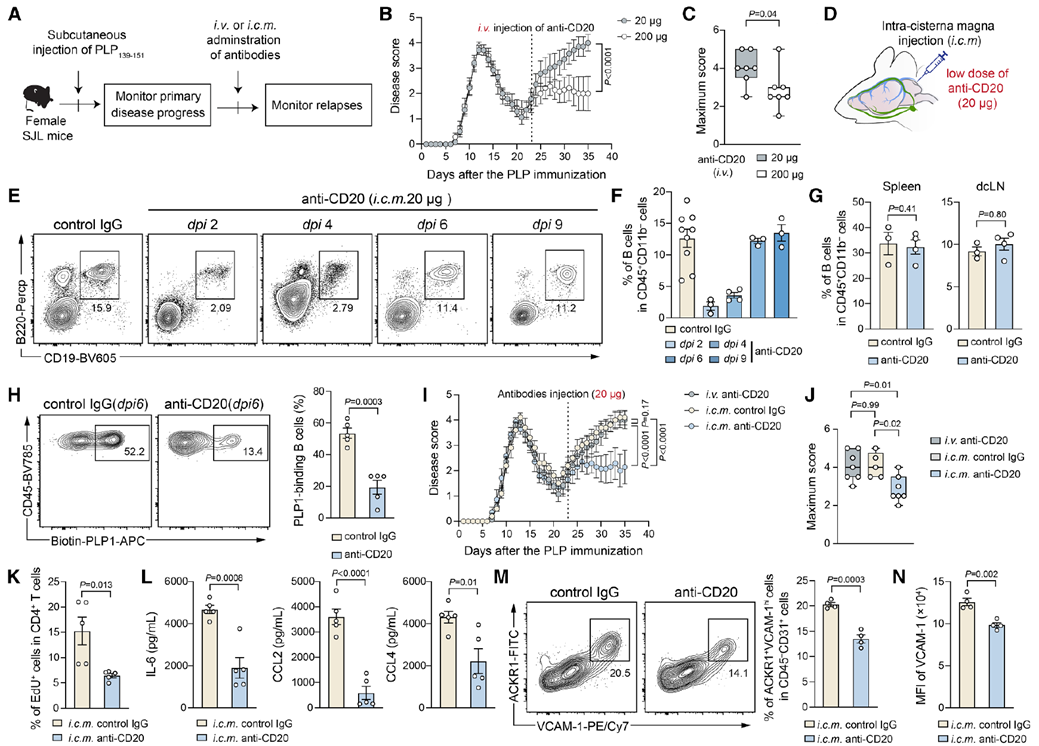
Figure 4. Targeted depletion of brain-localized B cells reduces EAE relapse
This study uncovers the molecular mechanism by which meningeal B cells trigger and amplify neuroinflammation through antigen presentation and cognate interactions with T cells. The findings demonstrate that selective depletion of brain-localized B cells can effectively mitigate relapses, offering new therapeutic avenues for MS. The recombinant murine MOG protein supplied by AtaGenix was central to establishing the model and validating these mechanisms, ensuring the rigor and reproducibility of the results. By filling a key gap in the understanding of MS pathogenesis, this work also provides a solid foundation for developing novel B-cell–targeted therapies, such as localized anti-CD20 or CD40 inhibitors.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

