AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-09-05 View volume: 664
Ovarian cancer is one of the most lethal malignant tumors of the female reproductive system, with recurrence and drug resistance posing significant challenges to clinical treatment. Studies indicate that ovarian cancer stem-like cells (OCSLCs) are key drivers of ovarian cancer progression and recurrence. However, the mechanisms maintaining OCSLC stemness remain unclear. The PI3K/AKT pathway is considered central to stem cell characteristics and drug resistance, but its specific regulation at the subtype level requires further exploration. A research team from China Medical University published an article in Advanced Science, focusing on the protein arginine deiminase family member PAD1 and its role in ovarian cancer stem-like cells.
PAD1 is upregulated in ovarian cancer and drives malignant phenotypes and stemness
Researchers first examined the transcriptional levels of the PAD family in human ovarian cancer tissues compared to ovarian cyst control samples, finding that PAD1 was significantly upregulated in tumors. Immunohistochemistry at the protein level also showed stronger PAD1 staining in tumor tissues, suggesting a correlation with tumorigenesis. In cell lines such as OVCAR3 and SKOV3, PAD1 was knocked down using lentiviral interference, confirming knockdown efficiency. This led to significant reductions in proliferation, migration, and invasion, as well as decreased tumor volume in nude mice, with IHC/WB confirming reduced PAD1 levels in tumors.
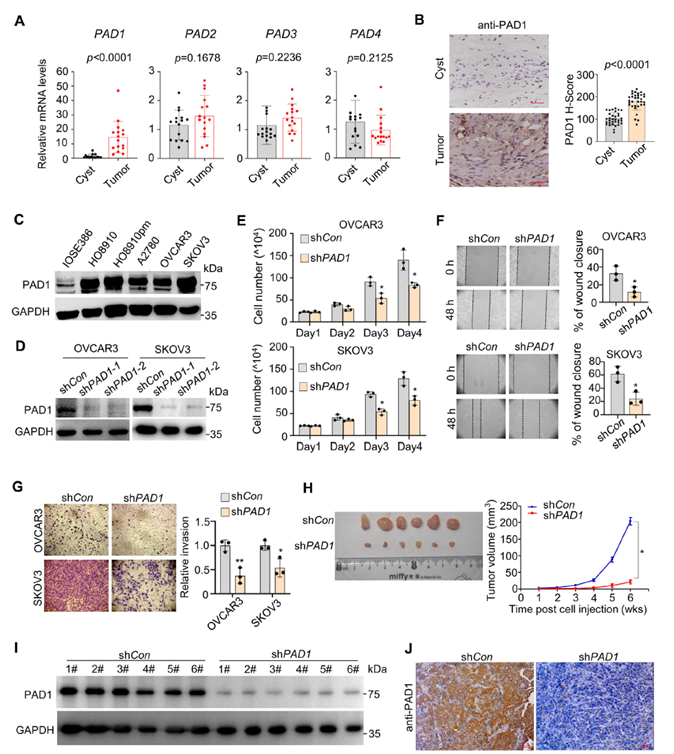
Figure 1. PAD1 expression is positively correlated with OC progression
Regarding stemness, PAD1 knockdown reduced the expression of CD133, CD44, OCT4, and SOX2, decreased sphere formation efficiency, and lowered tumor-initiating cell frequency in extreme limiting dilution assays (ELDA). Primary tumor cells derived from patient ascites also showed reduced stemness markers, sphere formation, and invasion capabilities following PAD1 interference.
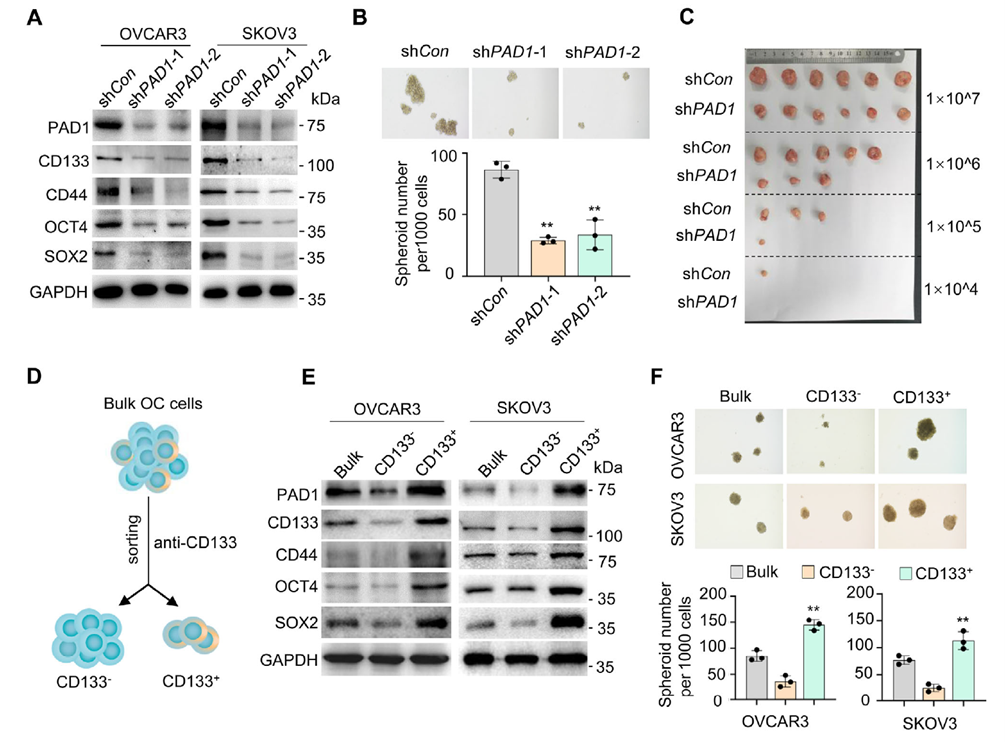
Figure 2. PAD1 enhances tumor-initiating capacity of OC cells
PAD1 maintains stemness via the AKT pathway and specifically interacts with AKT2
RNA-seq and enrichment analysis revealed that PAD1 interference led to differential gene expression enriched in the PI3K-AKT pathway. Pharmacological inhibition of PI3K/AKT (LY294002) dose-dependently reduced stemness markers. Western blot showed that PAD1 knockdown inhibited phosphorylation of key AKT activation sites, with CD133⁺ high-PAD1 subpopulations displaying higher AKT phosphorylation. Knocking down PAD1 in CD133⁺ cells significantly suppressed AKT phosphorylation. Mechanistically, co-immunoprecipitation and mass spectrometry indicated that PAD1 forms a complex with AKT2, with truncation and GST pull-down assays pointing to the AKT2 kinase domain as the interaction interface. Docking calculations further supported a high-affinity interaction between the two.
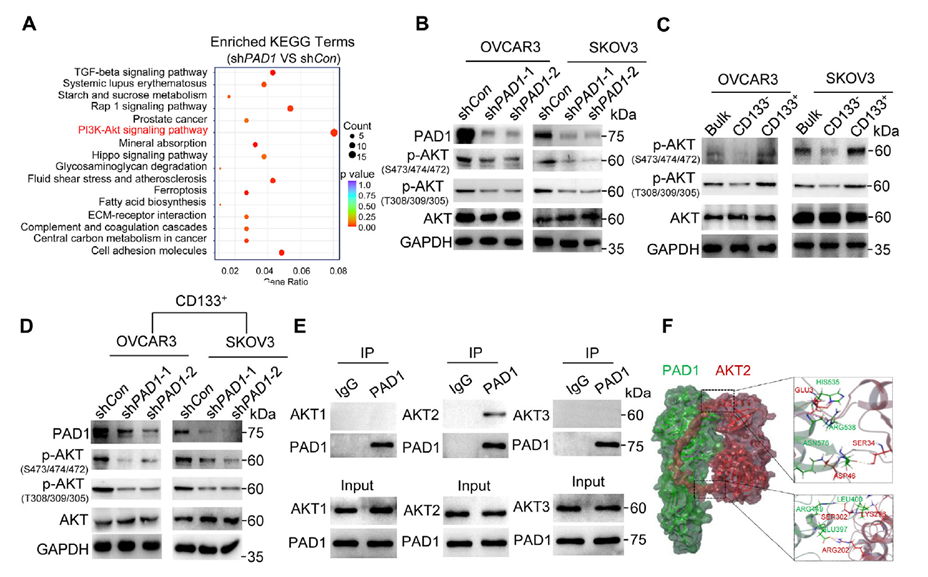
Figure 3. PAD1 maintains OCSLC stemness by activating the AKT signaling pathway
PAD1’s catalytic activity drives AKT2 activation: Evidence from D-Cl-amidine and in vitro citrullination
In HEK293 overexpression systems, PAD1 co-transfection increased AKT2 phosphorylation, while catalytically inactive mutants failed to activate AKT. The PAD1 inhibitor D-Cl-amidine (D-Cla) dose-dependently suppressed AKT phosphorylation in cells. In vitro reactions with purified proteins (His-AKT2 + rPAD1 + Ca²⁺) were recognized by anti-Pan-Cit antibodies, indicating direct citrullination of AKT2, dependent on Ca²⁺ and PAD1 catalytic activity. At the cellular level, overexpression of WT-PAD1 enhanced p-AKT2 immunofluorescence signals, while CS-PAD1 had no effect. Pharmacological PAD1 inhibition reduced stemness markers, suppressed sphere formation, and inhibited tumor growth and AKT2 activity/stemness markers in mouse xenografts. Combination with PI3K inhibitors showed additive suppression.
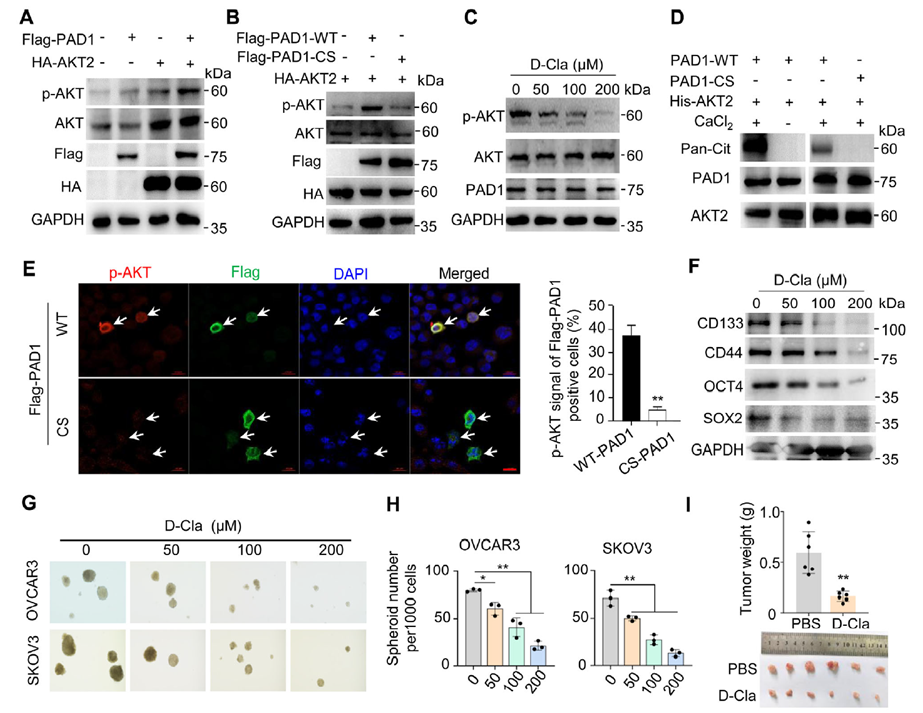
Figure 4. PAD1-catalyzed AKT2 citrullination promotes OCSLC stemness maintenance
AKT2 R202 citrullination is critical for kinase activity and phosphorylation site exposure
LC-MS/MS identified R202, R208, and R371 of AKT2 as citrullination sites (all located in the kinase domain) in in vitro reaction products. Researchers constructed R→E/K/A mutants to assess function: R202 mutation significantly reduced AKT2 phosphorylation, while most R208/R371 mutations had no effect. Structural modeling showed that only R202K disrupted surface exposure of S474 and T309, providing a structural explanation for impaired phosphorylation. Furthermore, a site-specific AKT2-Cit202 monoclonal antibody provided by AtaGenix detected R202 citrullination signals only in the presence of PAD1, both in vitro and in cells. Cit202 levels were significantly higher in multiple ovarian cancer cell lines compared to IOSE386, and clinical sample IHC showed enhanced Cit202 staining in tumor tissues.
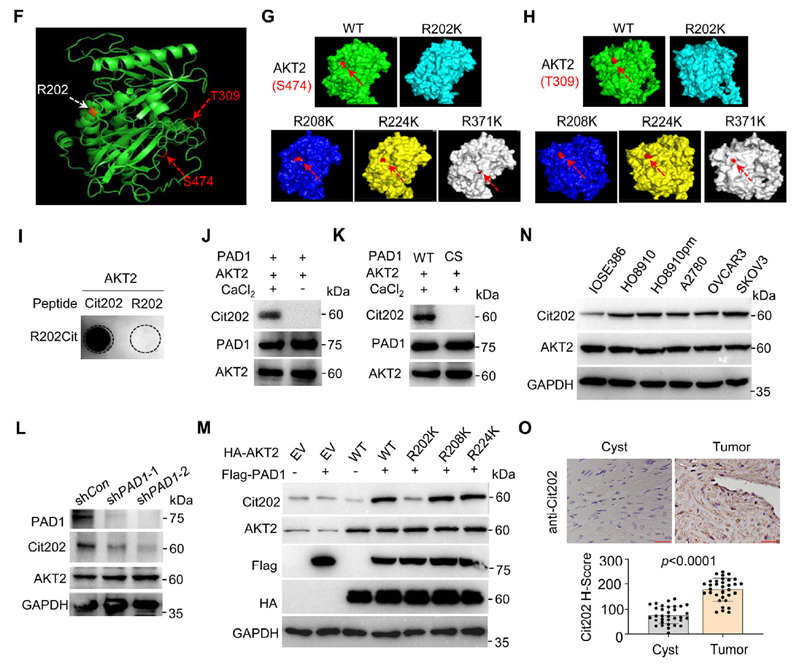
Figure 5. PAD1-mediated citrullination of AKT2 at R202 promotes AKT2 enzymatic activity in OC cells
R202K validation: Disrupting R202 citrullination replicates PAD1 knockdown’s “anti-stemness” phenotype
In OVCAR3 stable overexpression systems, AKT2-R202K significantly reduced AKT2 phosphorylation, R202 citrullination, and stemness marker expression compared to WT-AKT2. Sphere formation and invasion capacities decreased, and in ectopic xenograft models, peritoneal metastatic nodules and ascites volume were reduced. ELDA confirmed a decrease in CSC frequency, with p-AKT2 and stemness markers synchronously reduced in tumor tissues. These results closely mirrored the transcriptional profile of PAD1 knockdown, indicating that the R202 site is essential for the PAD1-AKT2 axis in maintaining stemness.
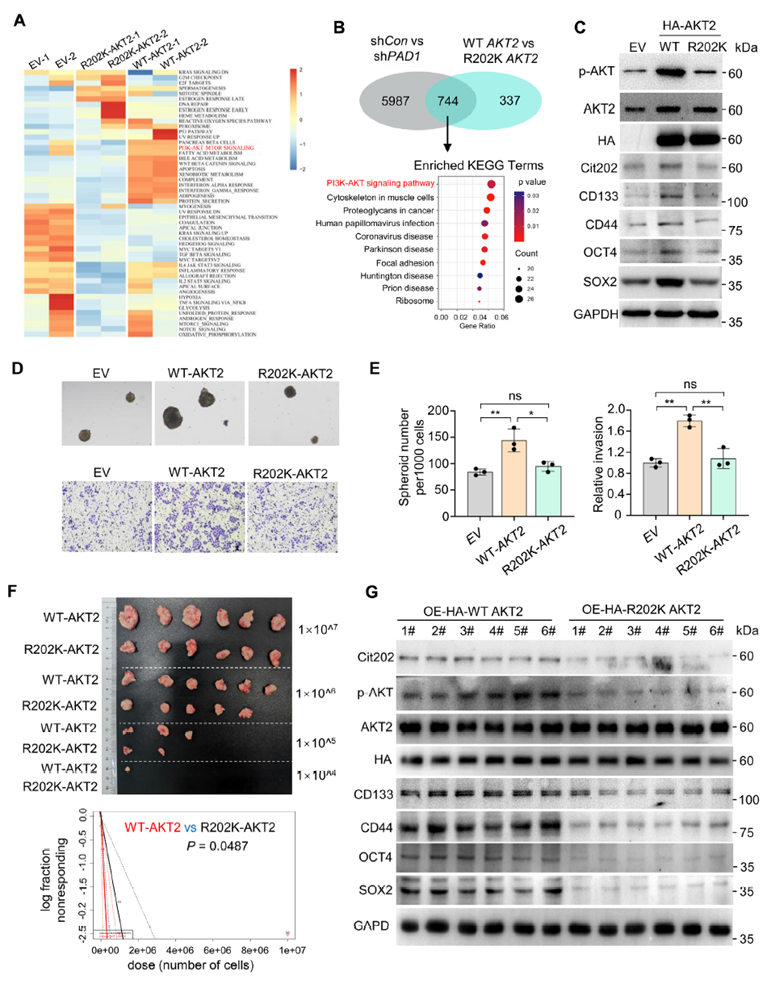
Figure 6. Overexpression of AKT2R202K in OC cells hinders OCSLC stem cell characteristics
CEBPβ as a key effector transcription factor of the PAD1-AKT2 axis
Promoter site scanning suggested that CEBPβ and YY1 may co-regulate SOX2/CD44/CD133/OCT4. ChIP-qPCR confirmed CEBPβ enrichment at the proximal promoters of these genes. PAD1 knockdown or AKT2-R202K overexpression significantly weakened CEBPβ binding, accompanied by reduced CEBPβ protein levels and stemness gene transcription. Pharmacological inhibition of PAD1 or PI3K/AKT dose-dependently reduced CEBPβ, with combined inhibition showing additive effects. In the presence of PAD1, R202 citrullination partially restored CEBPβ even under AKT2 S474A inactivation, suggesting that R202 citrullination may independently promote AKT2’s “favorable conformation/activity” to support CEBPβ upregulation.
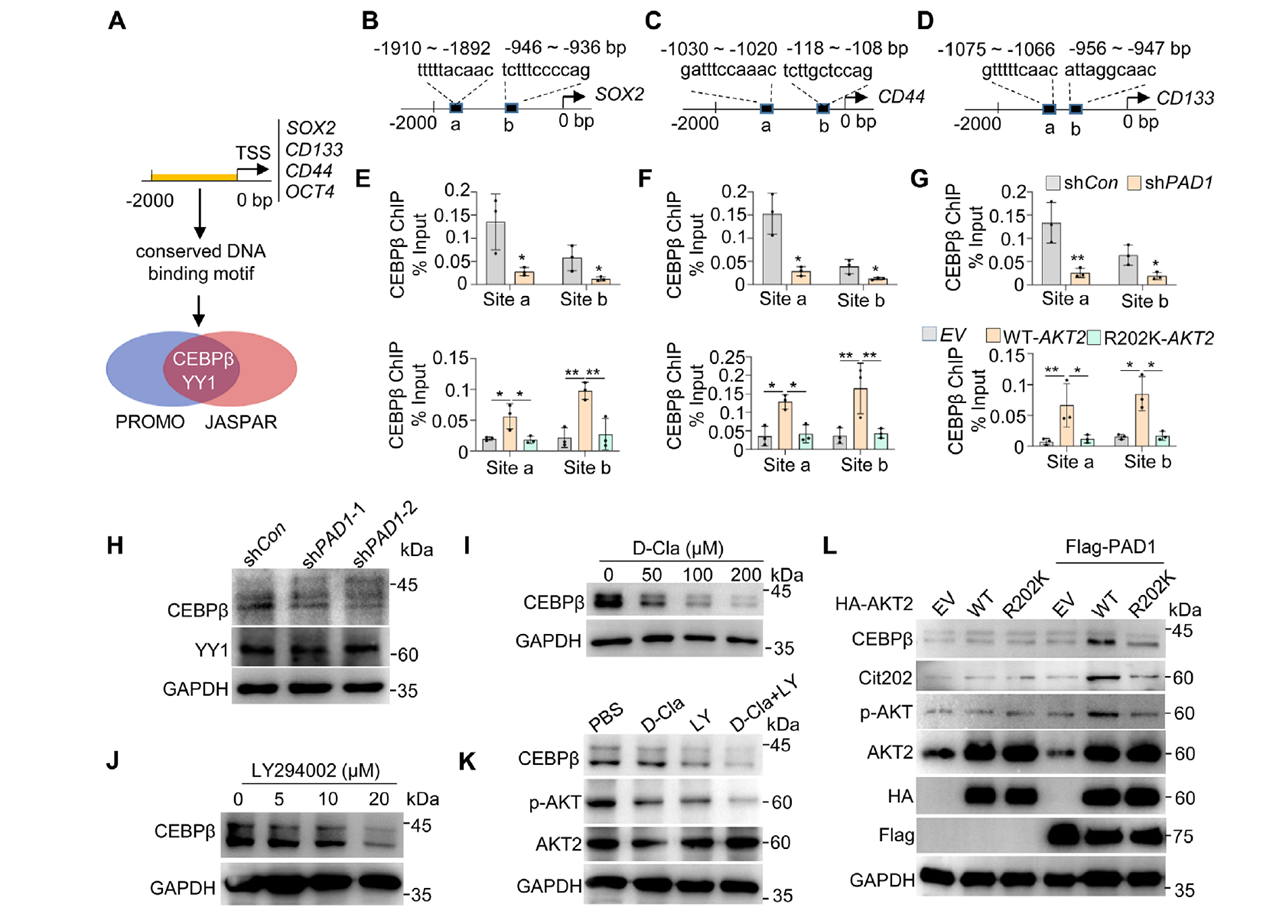
Figure 7. CEBPβ may serve as a key downstream regulatory target of the PAD1-AKT2 signaling pathway
PAD1 inhibition reverses cisplatin resistance and synergizes with AKT inhibitors
Researchers established OVCAR3-CisR cells (maintained with 5 μM cisplatin), which showed elevated stemness markers and enhanced sphere formation, accompanied by upregulated PAD1, enhanced AKT2-R202 citrullination, and increased p-AKT and CEBPβ, suggesting the PAD1-AKT2-CEBPβ axis correlates with resistance. D-Cla dose- and time-dependently inhibited AKT2 citrullination and phosphorylation in CisR cells, downregulating stemness molecules and suppressing proliferation, sphere formation, and invasion. Combination with AZD5363 (AKT inhibitor) showed additive inhibition. In nude mouse models, CisR tumors were insensitive to cisplatin alone, but D-Cla + cisplatin reduced tumor burden to levels comparable to the control group’s response to cisplatin. Tumor tissues showed significant reductions in p-AKT2, CEBPβ, and stemness markers, closing the loop on the “PAD1 inhibition → reduced AKT2-R202 citrullination → AKT2/CEBPβ inactivation → reversal of resistance” intervention pathway.
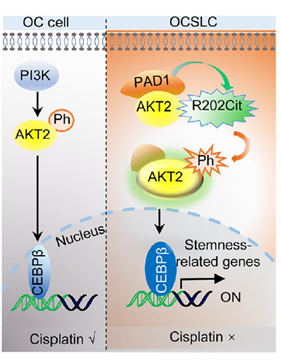
Figure 8. PAD1 inhibition re-sensitizes OVCAR3-CisR cells to cisplatin by suppressing the AKT2/CEBPβ signaling pathway
AtaGenix provided the site-specific AKT2-Cit202 monoclonal antibody for this study, playing a critical role in validating AKT2-R202 citrullination and its association with stemness and drug resistance both in vitro and in vivo. AtaGenix specializes in high-quality protein and antibody product development, offering one-stop services from antibody discovery and custom development to large-scale production, supporting researchers in advancing mechanistic studies and target validation.
To date, over 400 publications have cited AtaGenix’s one-stop protein and antibody development services. Looking ahead, AtaGenix will continue to support scientific innovation.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

