AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-08-28 View volume: 689
Patatin, the major glycoprotein in potato tubers, is known for its emulsifying, antioxidant, and lipid-modifying properties. With its nutritional value and versatile bioactivity, it has attracted growing interest as a plant-derived lipase. However, two limitations hinder its industrial application: low natural expression levels and a strong preference for short-chain substrates, leaving its activity toward long-chain fatty acids relatively weak.
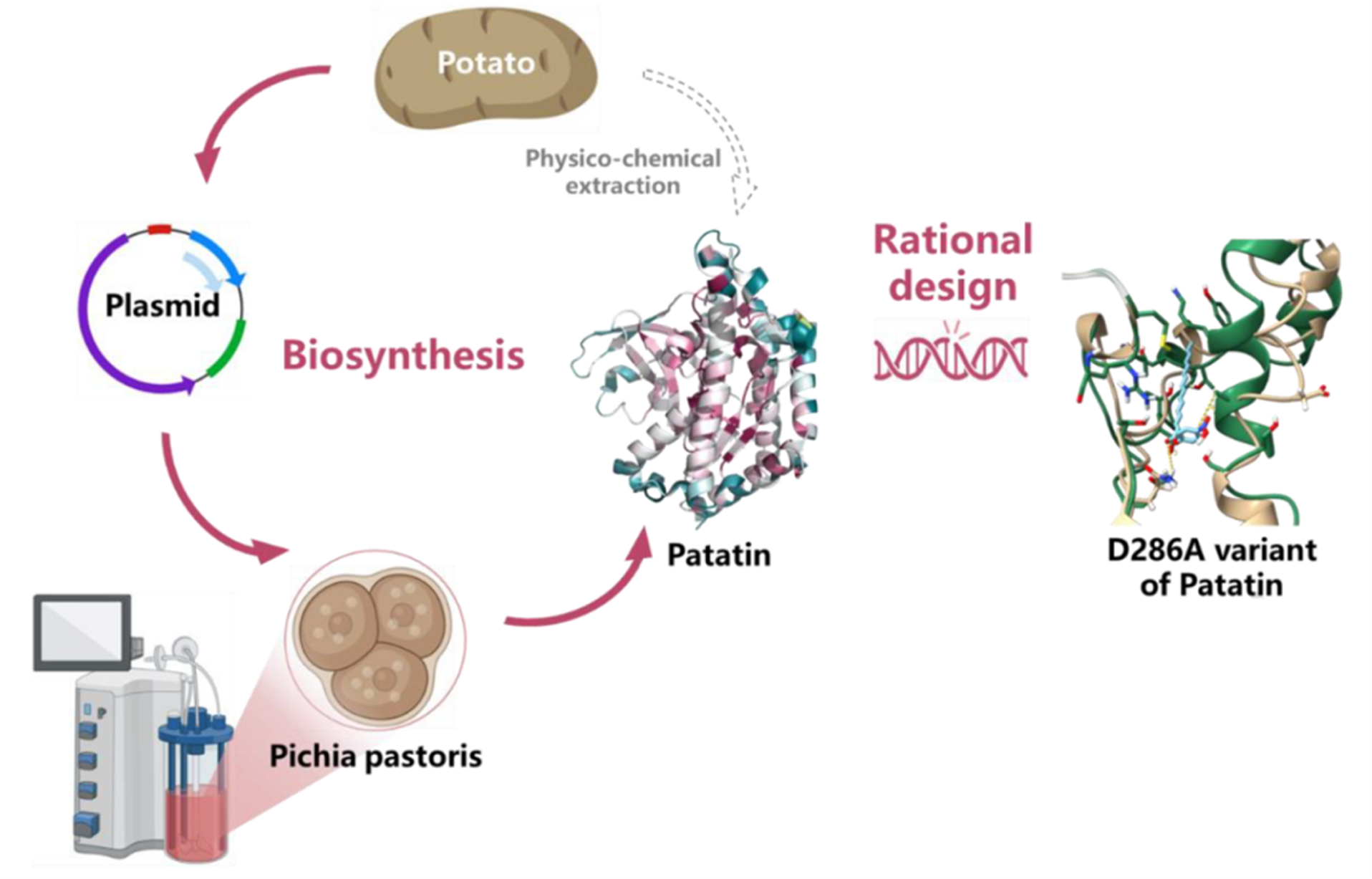
Figure 1. Biosynthesis of the D286A mutant
In this work, the research team successfully obtained recombinant Patatin using the Pichia pastoris expression system provided by AtaGenix, which enabled efficient protein production for subsequent functional studies and structural engineering. Enzymatic characterization showed that Patatin had an optimal temperature of around 35 °C and an optimal pH of 10.0, with good stability under alkaline conditions. Its activity was enhanced by methanol but strongly inhibited by Fe²⁺/Fe³⁺. While the enzyme displayed high activity toward short-chain substrates (such as pNP-C4), its catalytic efficiency for long-chain substrates (such as pNP-C16) remained limited.
To overcome this substrate preference, the researchers performed rational site-directed mutagenesis targeting residues near the substrate-binding pocket. Among the variants, the D286A mutant showed the most striking improvement:
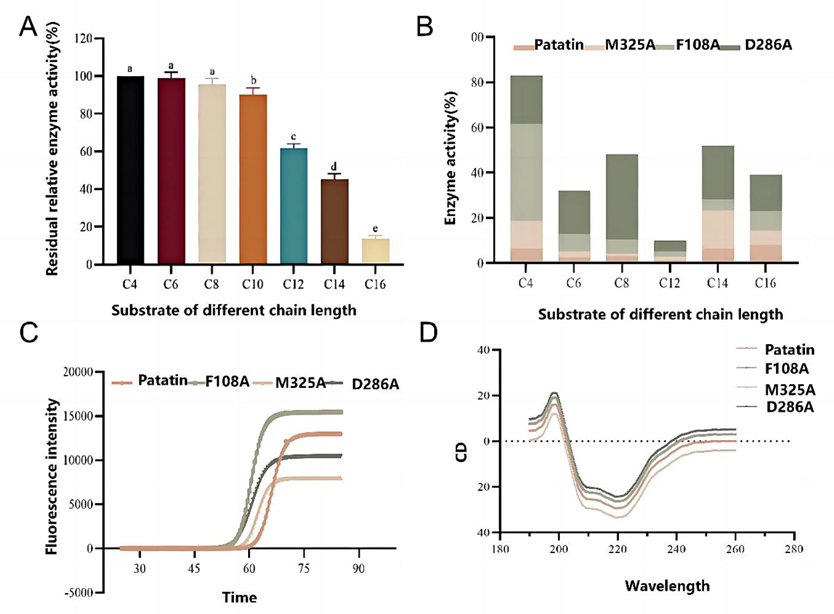
Figure 2. Substrate specificity of lipase Patatin
This study demonstrates, for the first time, that rational design can effectively shift Patatin’s substrate preference toward long-chain fatty acids, addressing a long-standing bottleneck in its application. The D286A variant not only expands the enzyme’s catalytic potential but also provides mechanistic insights into how subtle structural changes in local domains can fine-tune substrate specificity. By combining protein engineering with efficient expression, this work sets the stage for broader use of plant-derived lipases in functional lipid production, green biocatalysis, and food biotechnology.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

