AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2024-11-05 View volume: 828
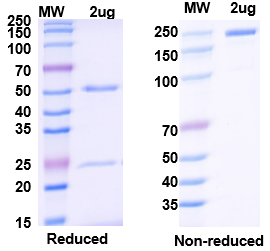
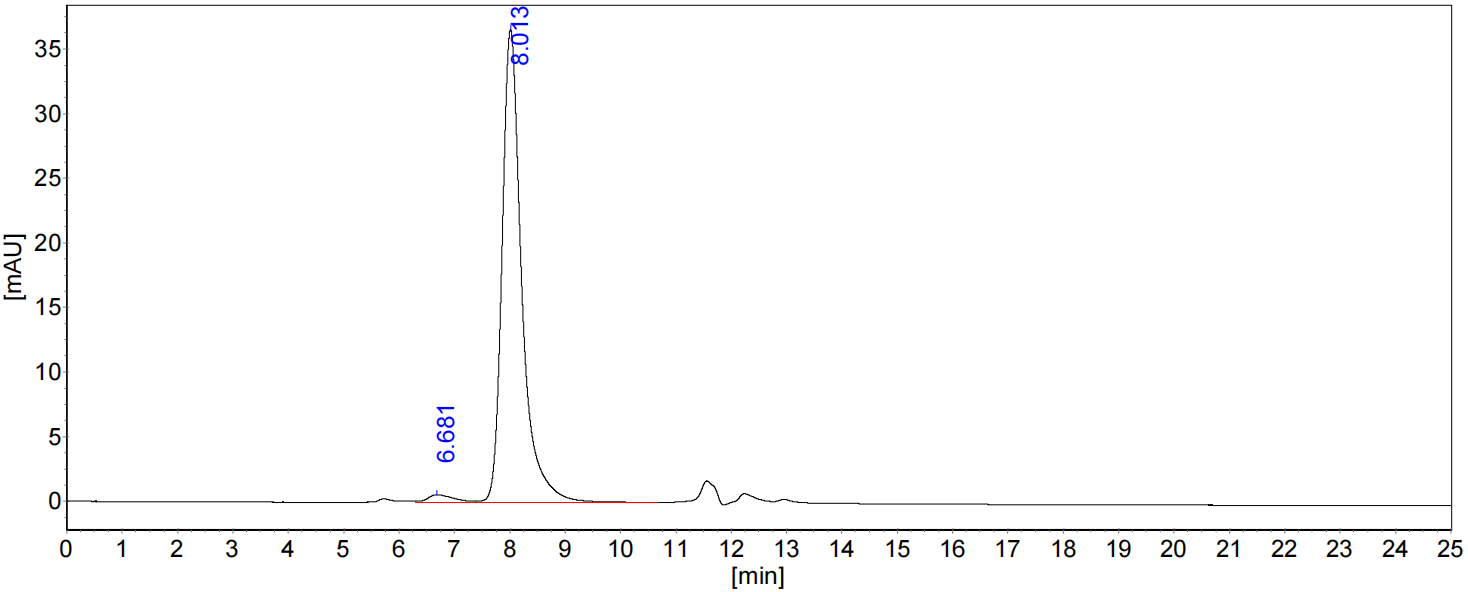
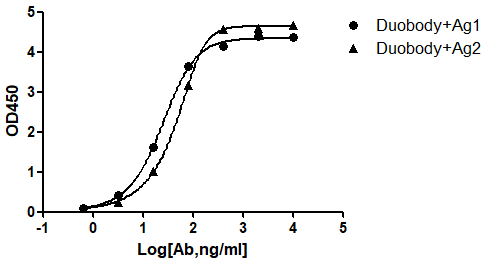
AtaGenix provides end-to-end bispecific antibody solutions — from recombinant expression and purification to QC validation — enabling faster, more reliable therapeutic development.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

