AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-08-20 View volume: 400
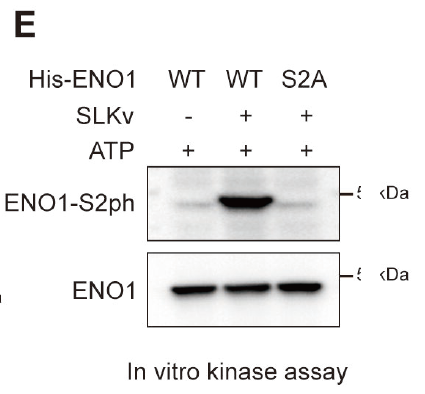
Abnormal energy metabolism in tumor cells is considered a key mechanism in promoting tumor initiation and progression, with the glycolysis pathway being particularly critical. This study focuses on the Ste20-like kinase (SLK) alternative splicing event, revealing that its variant SLKv enhances the production of phosphoenolpyruvate (PEP) by promoting phosphorylation at the S2 site of Enolase 1 (ENO1), thereby accelerating glycolysis and driving tumor development. Using samples from liver cancer patients, cell lines, and animal models, the research team systematically elucidated the mechanism of the “SLKv–ENO1–PEP” metabolic acceleration axis in tumors and proposed the potential therapeutic value of targeting this pathway.
To validate the critical function of the ENO1 S2 site, the study employed a highly specific phosphorylation antibody (customized by AtaGenix) to detect the modification status of ENO1. This tool enabled the research team to accurately confirm the phosphorylation level of ENO1, providing reliable experimental evidence for mechanistic analysis. The findings were published in Journal of Biological Chemistry (doi.org/10.1158/0008-5472.CAN-25-0523), offering new insights into tumor metabolic reprogramming.
Develop a highly specific phosphorylation antibody targeting the ENO1 Ser2 site for applications in WB, IP, IHC, and other scenarios; based on project progress, provide supporting services such as protein expression and purification, phage display screening, and functional validation.
Low immunogenicity of phosphorylation sites often leads to insufficient specificity; the antibody must be compatible with multiple detection platforms while maintaining low background; for subsequent protein function studies, stable and highly active recombinant proteins are required for control and validation.
1. Phosphorylation Antigen Strategy: Design phosphorylated peptides based on the Ser2 neighborhood sequence, optimize coupling and immunization protocols to enhance site specificity; deliver rabbit polyclonal/optional monoclonal solutions, including dephosphorylation adsorption to reduce cross-reactivity.
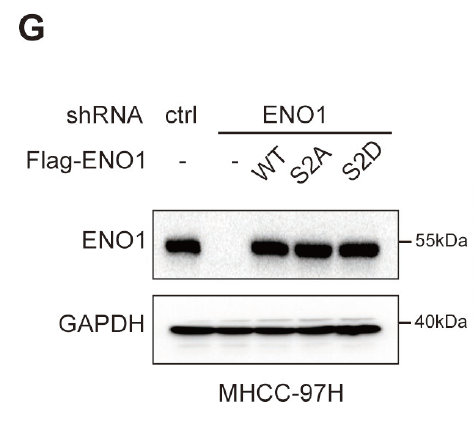
2. Protein Expression and Purification: Provide ENO1 and its mutants (S2A/S2D) expression in E. coli, insect, or mammalian systems, with multi-step purification (Ni-NTA, ion exchange, gel filtration) to ensure both purity and activity.
3. Phage Display: Construct and screen high-affinity antibodies/peptides for site recognition and supplementary validation in mechanistic studies.
4. Functional Validation and Quality Control: Provide validation data packages for WB, IP, ELISA, IF, IHC, SPR, and recommended usage conditions (dilution ratios, antigen retrieval, blocking conditions) to ensure cross-platform reproducibility.
The customized ENO1-S2 phosphorylation-specific antibody effectively supported the detection and mechanistic elucidation of site modifications, enabling the team to obtain reproducible molecular evidence at key nodes of the glycolysis pathway and providing a tool foundation for subsequent target validation and translational research.
For more information, visit www.atagenix.com. AtaGenix provides one-stop customized support from antibody/protein design to functional validation, covering custom antibodies, protein expression, phage display, and multi-platform validation.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

