AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2024-11-05 View volume: 877

Figure 1: Schematic of antibody coding gene structure, including 5’ and 3’ untranslated regions (UTRs). This illustrates the framework of heavy and light chain organization.
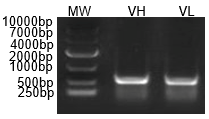
Figure 2: PCR amplification of antibody heavy (VH) and light chain (VL) variable regions, showing clear amplification bands, confirming the successful cloning of variable regions for sequencing.

Figure 3: Colony PCR validation gel. M: DL10000 DNA Marker. The clear in-frame amplification bands confirm successful insertion of VH and VL sequences into the cloning vector.
| Sequence | V-GENE and Allele | Functionality | V-REGION Identity (%) | J-GENE and Allele | D-GENE and Allele | AA Junction | Junction Frame |
|---|---|---|---|---|---|---|---|
| VH | Musmus IGHV1-71-16*01 F | Productive | 96.53% | Musmus IGHJ4*01 F | Musmus IGHD2-4*01 F | CAITTGYAMDYW | In-frame |
| VL | Musmus IGKV4-74*01 F | Productive | 97.51% | Musmus IGKJ4*01 F | - | CHQYYRSPFTF | In-frame |

Figure 4: Antibody heavy and light chain sequence analysis. High sequence identity (>96%) confirms accurate cloning and alignment of VH and VL regions.

Figure 5: Sequencing peak map of antibody VH and VL chains. Clear and consistent peaks demonstrate sequencing accuracy and reliable antibody variable region identification.
Need expert support for antibody cloning, sequencing, and validation? AtaGenix delivers end-to-end custom antibody services—from design to QC.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

