AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-06-20 View volume: 311
Study Context
Herpes simplex virus 1 (HSV-1) is a globally prevalent virus causing mucocutaneous lesions and, in rare cases, encephalitis. A 2025 study published in Vaccine (DOI: 10.1016/j.vaccine.2025.127241) by researchers at the Chinese Academy of Medical Sciences (Kunming) investigated the immunogenicity of HSV-1 fusion glycoprotein B (gB) and its H516P prefusion mutant in mRNA and subunit vaccines. The study found no significant difference in immunogenicity between gB and gB H516P, highlighting the potential of subunit vaccines with QS-21 and CpG ODNs adjuvants to induce robust cellular immune responses critical for controlling latent HSV-1 infections.Contact AtaGenix for Vaccine Protein Solutions →

Client Requirements
The research team, led by Jingping Hu and colleagues at the Chinese Academy of Medical Sciences (Kunming), required high-purity gB and gB H516P proteins for immunological assays, including enzyme-linked immunospot (ELISpot) and enzyme-linked immunosorbent assay (ELISA), to evaluate vaccine-induced immune responses in mice. AtaGenix Laboratories Co., Ltd. (Wuhan, China) was commissioned to provide custom protein expression and purification services using a mammalian cell expression system, ensuring compatibility with ELISpot validation and other functional assays.Explore AtaGenix Custom Protein Services →
Technical Challenges
1. Protein Purity: Achieving high-purity gB and gB H516P proteins for accurate ELISpot and ELISA results.Partner with AtaGenix for High-Purity Vaccine Proteins →
Custom Protein Solution
AtaGenix collaborated with the Chinese Academy of Medical Sciences (Kunming) to deliver custom protein expression services for their HSV-1 vaccine study published in Vaccine (DOI: 10.1016/j.vaccine.2025.127241). Our solution included:1. Protein Expression and Purification:
- Expressed gB and gB H516P proteins using Chinese hamster ovary (CHO) cells in a mammalian cell expression system for optimal post-translational modifications.
- Purified proteins to high purity using a nickel column, ensuring suitability for ELISpot, ELISA, and other immunological assays.
2. Validation and Support:
- Validated protein functionality through ELISpot assays, confirming antigen-specific T-cell responses and protein integrity.
- Provided technical support to optimize protein performance in ELISA and ELISpot assays, enabling precise measurement of gB-specific IgG titers and cytokine production (IL-2, IFN-γ).
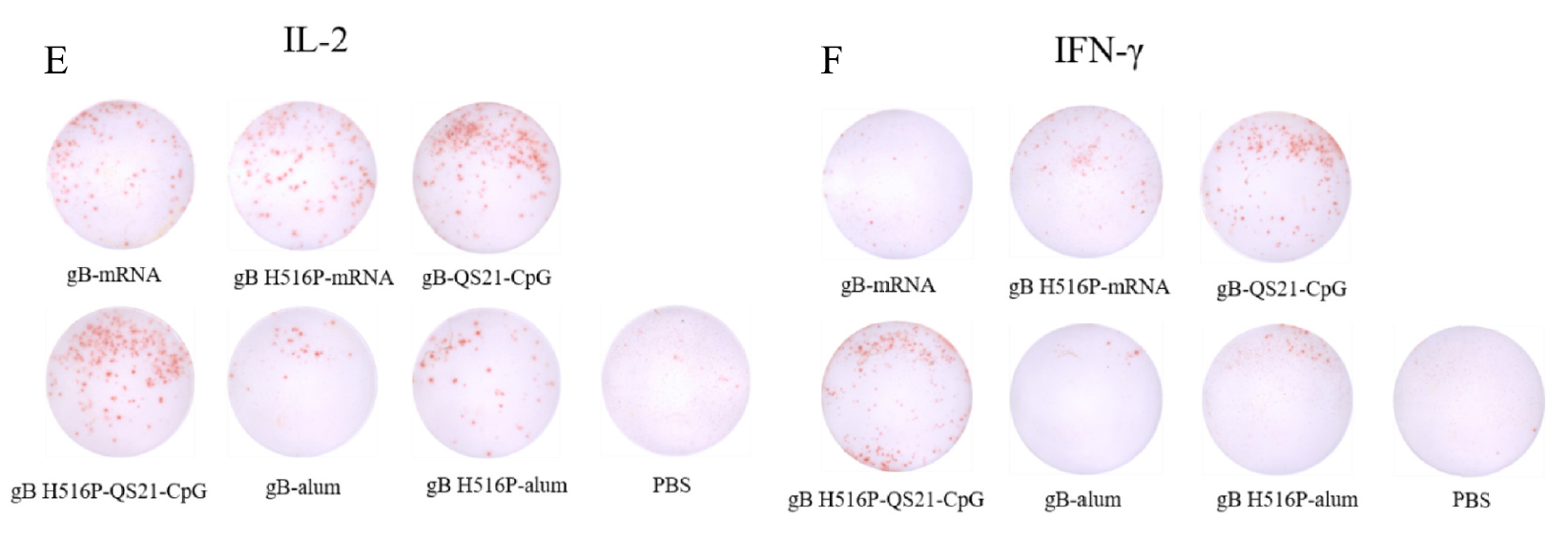
The high-purity gB and gB H516P proteins enabled the research team to confirm comparable immunogenicity between wild-type and mutant proteins, supporting the study’s findings on the efficacy of QS-21 and CpG ODNs adjuvants in subunit vaccines. Validated through ELISpot, the proteins advanced the understanding of cellular immune responses critical for HSV-1 vaccine development.
Learn More About AtaGenix Vaccine Protein Solutions →
For more information, visit Contact AtaGenix for Protein Expression Solutions →
AtaGenix provides one-stop customized support from antibody/protein design to functional validation, covering custom antibodies, protein expression, phage display, and multi-platform validation.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

