AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-05-30 View volume: 326
Customer Needs
The study required the heterologous expression and purification of PgUGT29 and PgUGT72 proteins in E. coli to validate their enzymatic functions, alongside the development of specific antibodies to study protein interactions and confirm glycosylation activity at the C3 position of PD, supporting the elucidation of saponin biosynthesis pathways.
Technical Challenges
1. Protein Expression and Purification: Plant-derived UGT proteins like PgUGT29 and PgUGT72 often exhibit low solubility and misfolding in E. coli, necessitating optimized expression conditions and refolding strategies to achieve high purity (>95%) and functional activity.
2. Antibody Specificity: Developing antibodies against PgUGT29 requires designing antigens targeting specific epitopes to ensure high specificity and minimal cross-reactivity, critical for accurate detection in complex assays like Western blot and immunofluorescence.
3. Functional Validation: Ensuring the recombinant UGT proteins retain enzymatic activity in in vitro assays (e.g., HPLC-based glycosylation assays) and that antibodies accurately detect target proteins in molecular studies demands rigorous quality control and validation.
Technical Service Solutions
At AtaGenix, we provide tailored protein expression and antibody customization services to support P. grandiflorus UGT research:
1. Recombinant PgUGT Expression and Purification:
(1) Express His-tagged PgUGT29 and PgUGT72 in E. coli BL21 (DE3) using IPTG induction (1 mM, 37°C, 4 h), followed by Ni-NTA affinity chromatography and urea dialysis refolding, achieving protein purity >95% and concentrations of 0.21–0.50 mg/mL, verified by SDS-PAGE and Western blot.
(2) Quantify proteins using the Bradford method to meet HPLC assay requirements for enzymatic activity testing.
2. Anti-PgUGT29 Antibody Customization and Validation:
(1) Design antigens targeting PgUGT29-specific epitopes (e.g., PSPG motif regions) to produce rabbit-derived polyclonal antibodies, validated for specificity via Western blot and immunofluorescence to ensure accurate detection of PgUGT29 in plant tissues.
(2) Deliver antibodies within 4–6 weeks to align with project timelines.
3. Functional Validation Support:
(1) Provide HPLC-based enzymatic assays to confirm PgUGT29’s glycosylation activity (PD to PD3) using UDP-Glc as the donor, with negative controls (e.g., PgUGT72) to ensure specificity.
(2) Support molecular docking studies to identify key residues (e.g., T145, D392, Q393, N396) for UDP-Glc binding, enhancing understanding of enzyme-substrate interactions.
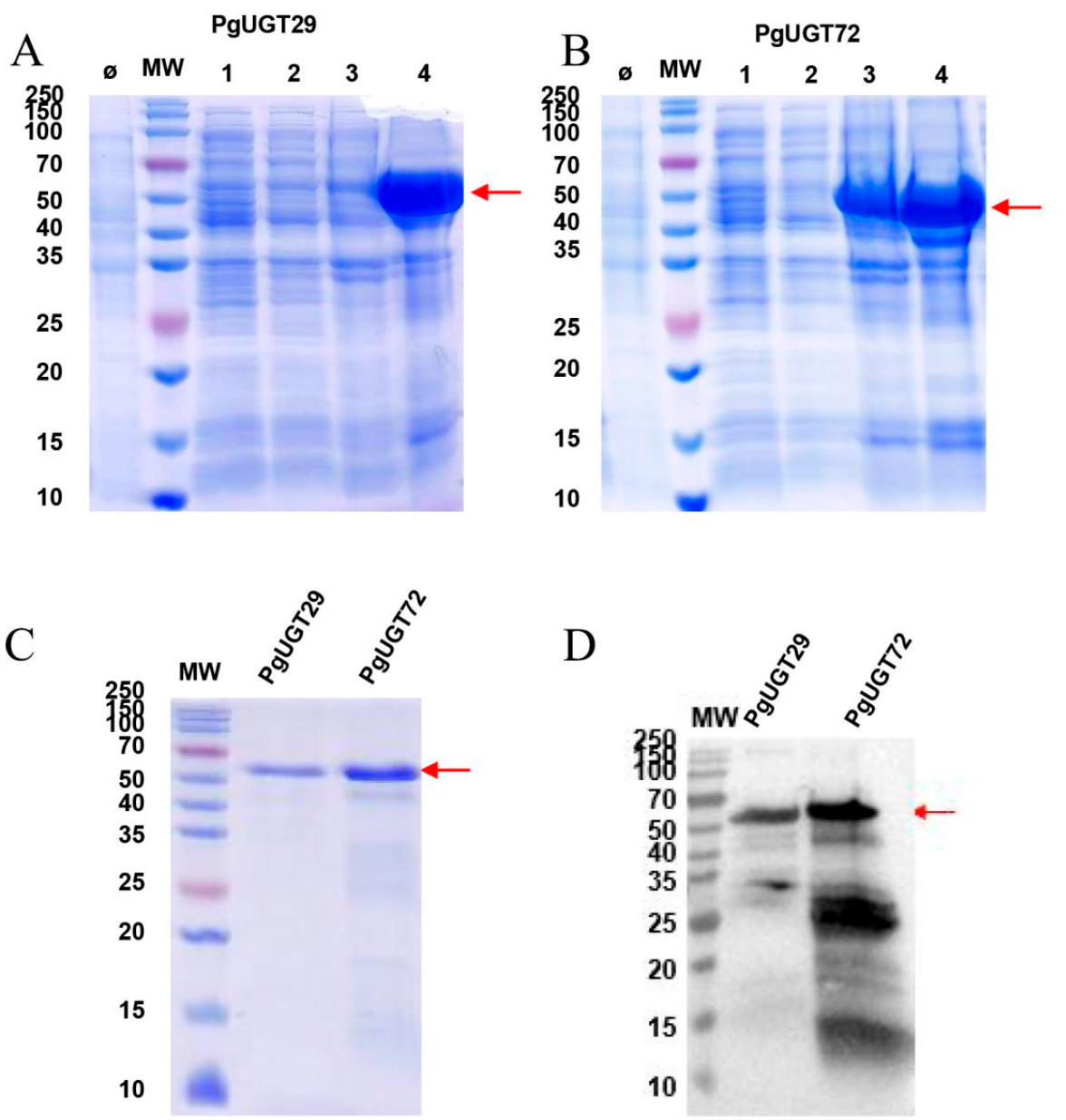
Our protein expression and antibody customization services enabled the successful expression and purification of PgUGT29 and PgUGT72, with PgUGT29 confirmed to catalyze PD to PD3 conversion, as validated by HPLC. The anti-PgUGT29 antibodies demonstrated high specificity in detecting the target protein, facilitating precise functional studies. These results support the elucidation of the saponin biosynthesis pathway in P. grandiflorus, achieving a significant milestone in metabolic engineering with a potential yield increase of bioactive saponins (e.g., PD3) for pharmaceutical applications.
For more information, visit www.atagenix.com. AtaGenix provides one-stop customized support from antibody/protein design to functional validation, covering custom antibodies, protein expression, phage display, and multi-platform validation.
Need recombinant UGT expression, antibody development, or HPLC activity validation for plant metabolic engineering? Talk to AtaGenix experts.
Contact AtaGenix for Custom SolutionsContact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

