AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2024-10-21 View volume: 638
The XtenCHO™ high-density expression system from AtaGenix is designed to maximize both cell growth and antibody yield in therapeutic development. In 15-day culture runs, CHO cells consistently maintained high density and near-100% viability, creating a stable environment for antibody production. Yields across multiple molecules confirmed the platform’s adaptability, supporting reliable and scalable biomanufacturing.
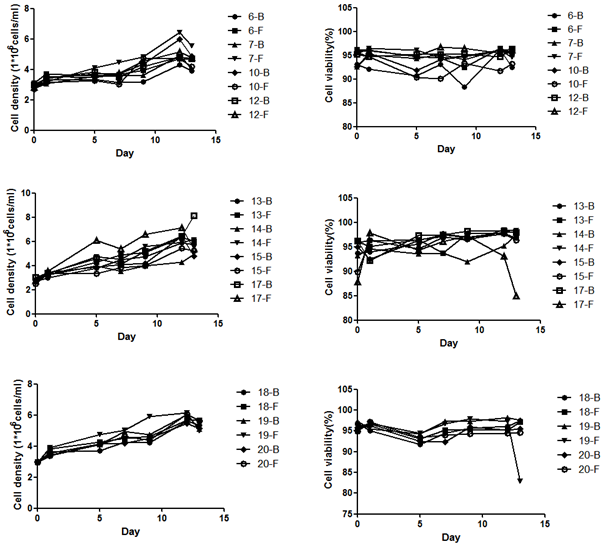
Cell density: Most cell lines expanded steadily, reflecting excellent proliferation and optimized growth conditions. Differences between clones highlight natural variability in expression constructs and line-specific metabolism.
Cell viability: Viability remained close to 100% throughout most of the culture period. A slight decline in select lines near day 15 is common under long-term culture, often linked to nutrient depletion or waste accumulation. These effects can be managed through optimized feeding strategies and process control.

Antibody yield distribution: Most molecules reached 500–1000 mg/L, while the best performer approached ~1500 mg/L. A few outliers remained below 100 mg/L, reflecting structural or sequence-specific challenges. These can typically be improved with targeted optimizations, such as leader peptide tuning or process adjustments.
Process implication: The consistent mid-to-high yields across diverse antibody types demonstrate that XtenCHO™ is broadly compatible and scalable, helping researchers shorten development cycles and reduce production risks.
Accelerate therapeutic antibody development with consistent high-density growth and strong yields. Contact AtaGenix for XtenCHO™ process development and optimization support.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

