AtaGenix Laboratories
AtaGenix Laboratories
2024-11-05
Protein A and Protein B Expression in Bacillus subtilis | High-Purity Recombinant Protein Solutions by AtaGenix
Discover how AtaGenix optimized Protein A secretion and Protein B intracellular expression in Bacillus subtilis. Case studies show high yields and >90% purity validated by SDS-PAGE, highlighting AtaGenix’s expertise in recombinant protein expression and quality control.
2024-11-05
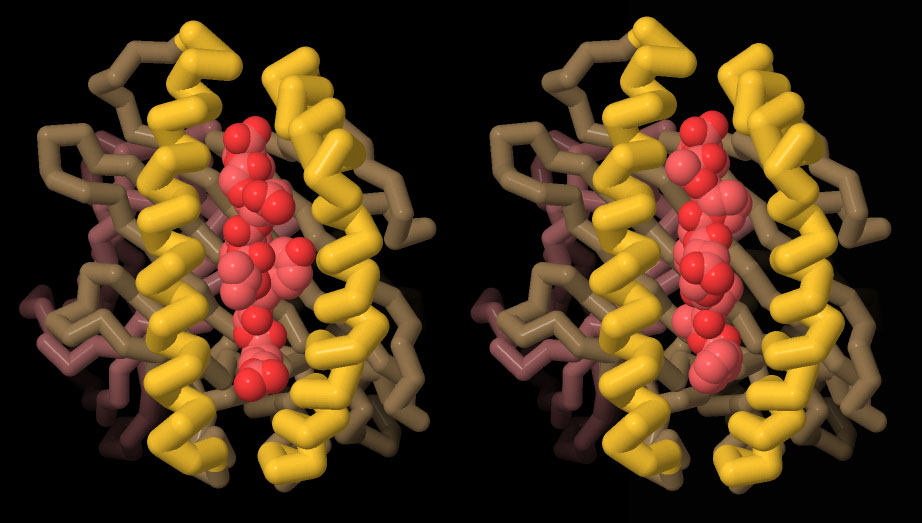
HLA-I Expression and pMHC Tetramer Assembly in E. coli | AtaGenix
HLA-I protein and β2M were expressed and purified in E. coli, followed by refolding to form stable HLA/β2M/peptide complexes (pMHC). The addition of streptavidin (SA) enabled the formation of stable tetramers for downstream applications.
2024-11-05
Protein Expression & Case Studies | AtaGenix Recombinant Protein Solutions
This study demonstrates the optimization of E. coli expression systems for recombinant protein and antibody fragment production. Strategies included host strain selection, periplasmic expression for proper folding, and refolding inclusion bodies into active soluble proteins. Results showcase high-quality protein expression and QC validation.
2024-11-05
Antibody Gene Sequencing and Validation Case Study | AtaGenix Custom Antibody Development
Hybridoma sequencing enables the precise identification of heavy and light chain variable regions of monoclonal antibodies. The data includes high-quality PCR amplification, colony validation gels, and sequencing results, ensuring accuracy in gene identification and antibody production.

2024-11-04
Recombinant Antibody Expression | AtaGenix Custom Antibody Services
This case study demonstrates the use of the insect-baculovirus expression system for efficient production of recombinant proteins. The approach achieved high yields with proper folding and post-translational modifications, showcasing its reliability for complex protein expression.

2024-11-04
Xten™ Mab Single B Rabbit Monoclonal Antibody Development Case Study
Using rabbit single B-cell antibody technology, an Anti-human CD86 rabbit monoclonal antibody was developed. Rabbits with serum titers greater than 128K were selected, and PBMCs were isolated for flow sorting to obtain human CD86 antigen-specific B-cell plates.

2024-11-04
SARS-CoV-2 Spike Protein Expression & Purification | AtaGenix High-Yield Mammalian System
The results show that the SARS-CoV-2 S protein expressed in mammalian cells achieved high yields during purification, with clear target protein bands observed in elution fractions (E1-E8) and minimal impurities.

2024-10-21
XtenCHO™ High-Density Expression System
AtaGenix's XtenCHO™ high-density expression system demonstrates exceptional proliferation and yield advantages in therapeutic antibody production. Cells cultured in this system maintain high density and viability over 15 days, ensuring healthy growth. The system shows strong adaptability and production efficiency across various antibodies, providing robust support for efficient and stable therapeutic antibody manufacturing.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

