AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2024-11-05 View volume: 779
Case 1:
1. Expression system optimization included host strains, induction temperature, induction time, inducer concentration, and culture media.
| Host Strains | Induction Temperature | Induction Time | Inducer | Culture Media |
|---|---|---|---|---|
| T7E, BL21, C41, Arctic | 16°C / 37°C | 16h / 4h | IPTG | LB / Auto-induction medium |
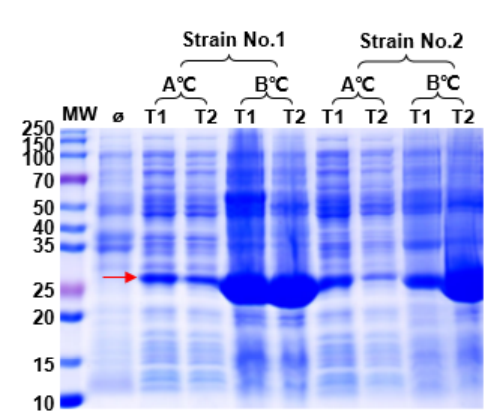
Fig. 1: Optimization of Protein Expression Conditions
Optimized conditions improved solubility and minimized impurities across different host strains.
2. 3C enzyme was used to remove the His-tag from the target protein. QC results are shown below:
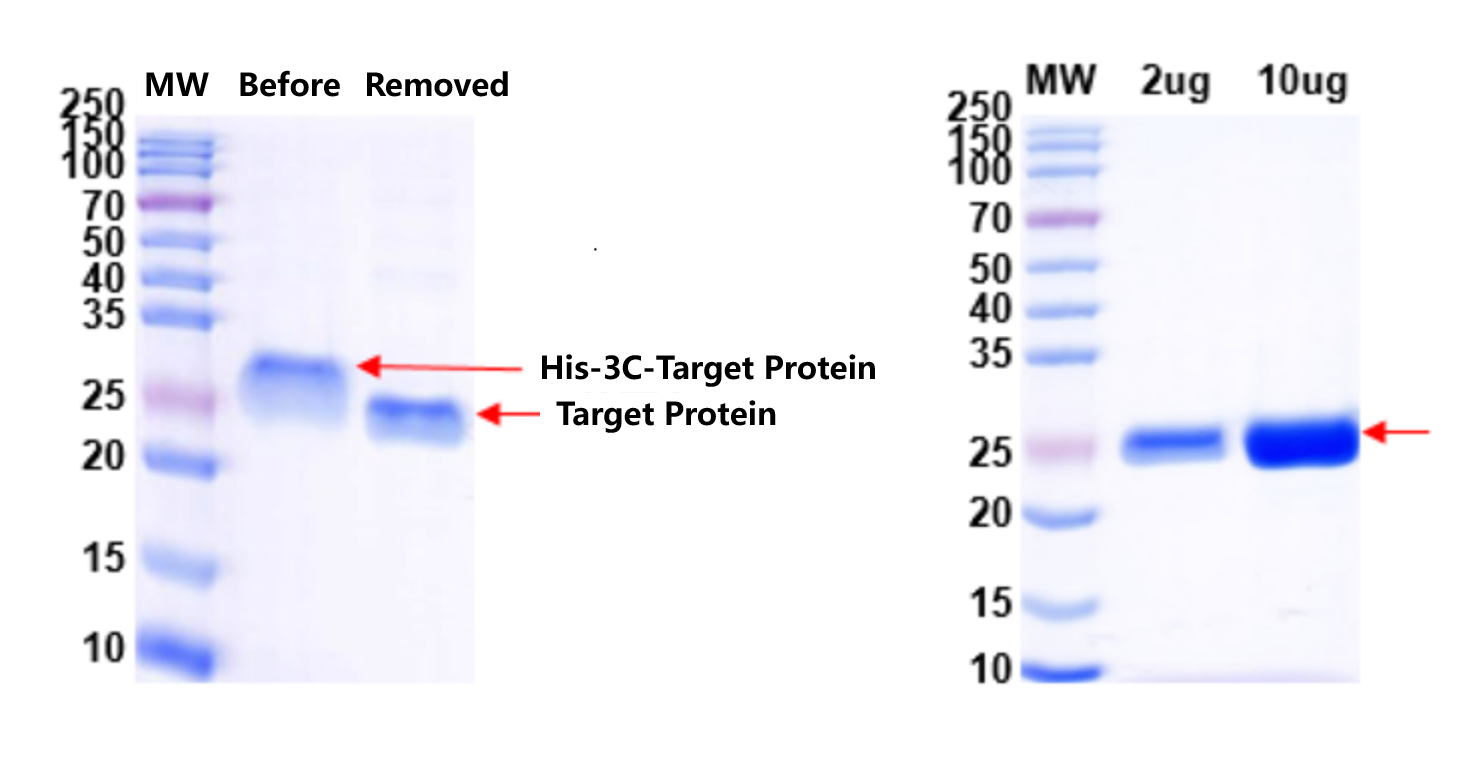
Fig. 2: Removal of His-tag with 3C Enzyme Fig. 3: QC of His-tag Removed Protein
His-tag was efficiently cleaved, and QC confirmed target integrity with minimal by-products.
Case 2:
Antibody fragments expressed in E. coli often misfold due to disulfide bonds, forming inclusion bodies. By directing expression to the periplasmic space, properly folded soluble fragments were obtained.
1. Protein expression and QC results are shown below:
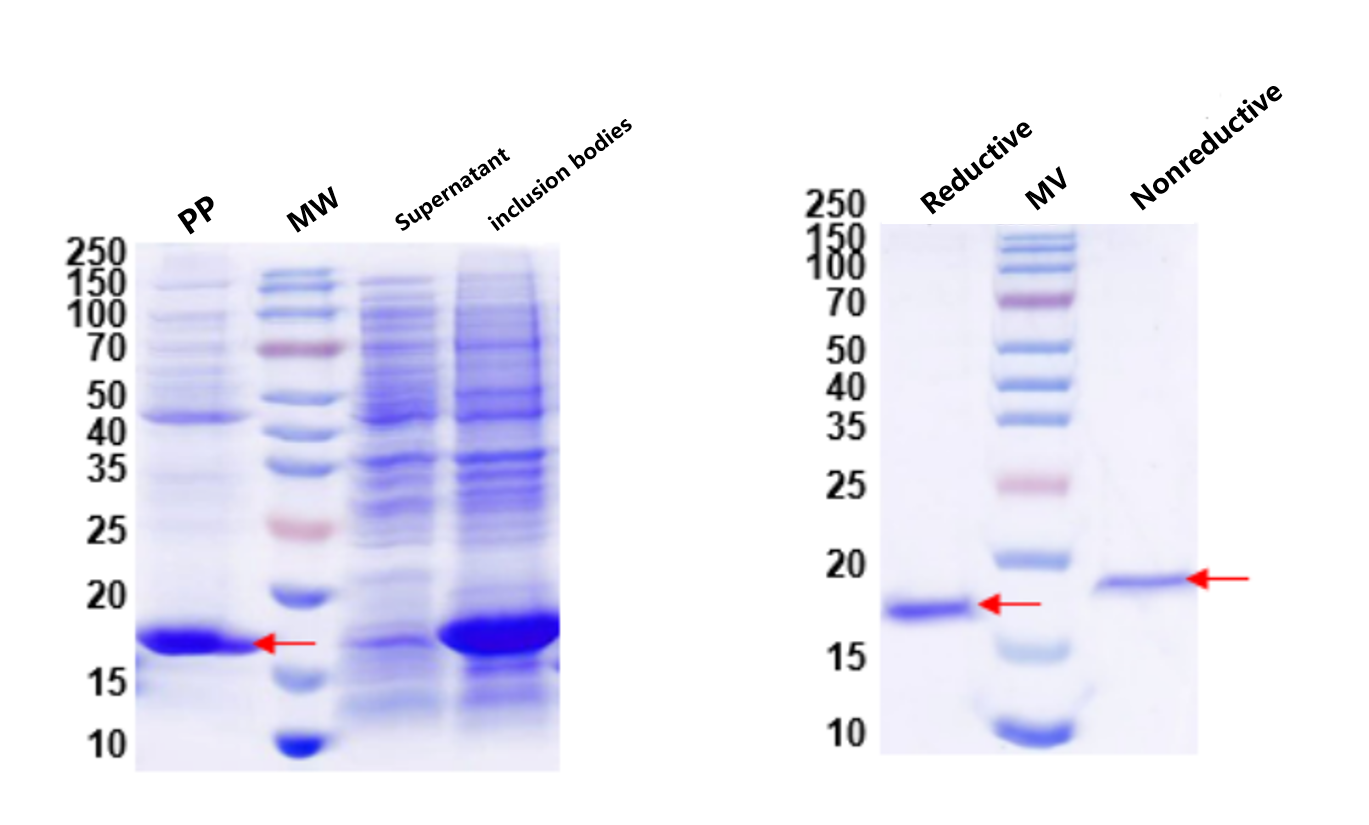
Fig. 4: Periplasmic Expression of Antibody Fragment Fig. 5: QC (Reduced / Non-reduced)
QC confirmed correctly folded soluble antibody fragments distinct from aggregated inclusion bodies.
Lane MW: Protein marker | Lane PP: Periplasmic Space
Case 3:
Many proteins in E. coli form inclusion bodies. To restore functionality, these were refolded into soluble proteins for activity assays.
1. Expression and QC results are shown below:
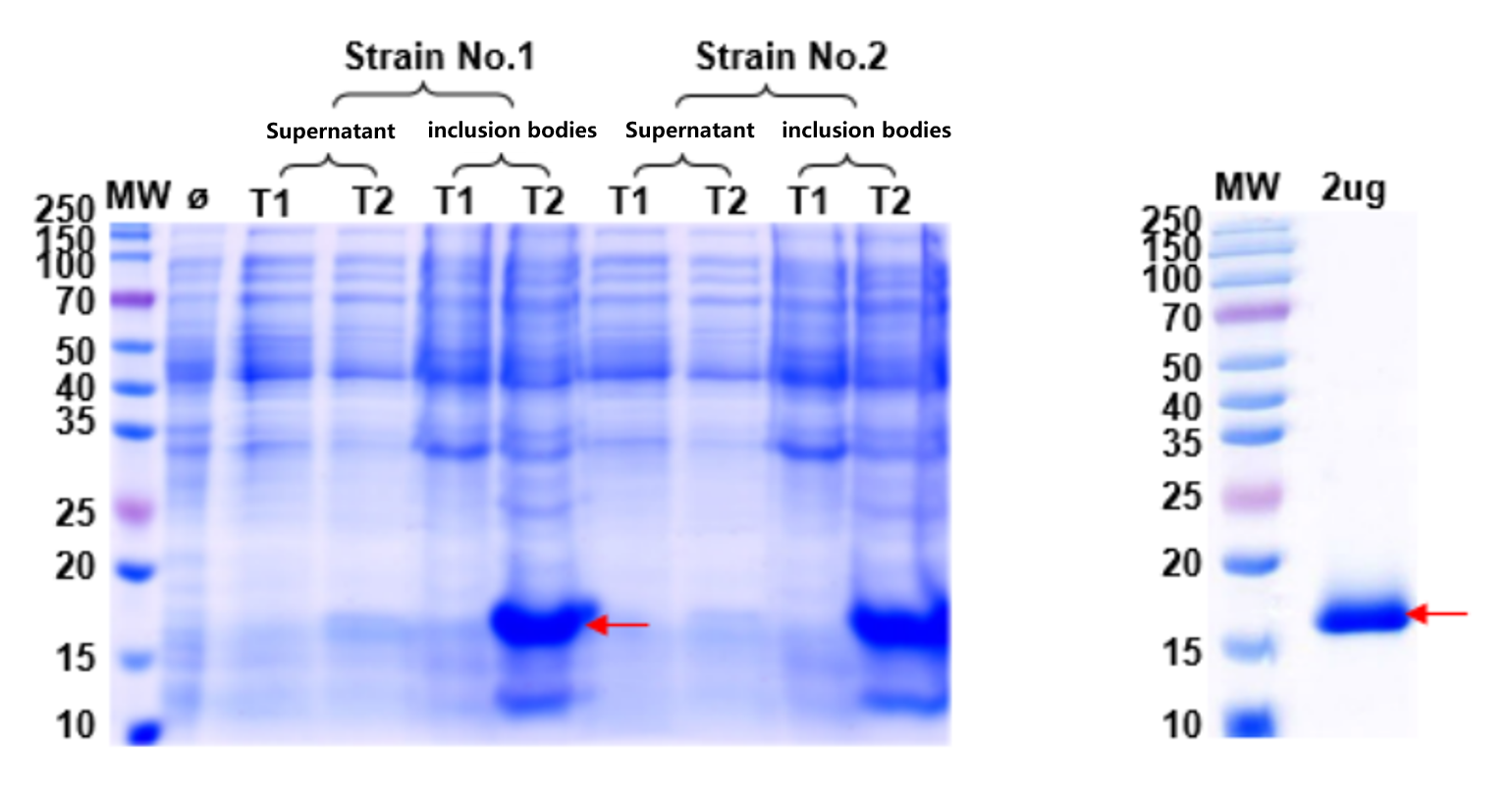
Fig. 6: Optimization of Target Protein Expression Fig. 7: Refolding QC of Target Protein
Refolding successfully converted insoluble inclusion bodies into soluble, functional proteins validated by QC bands.
Need optimized protein expression and validation support? AtaGenix provides end-to-end custom solutions for antibody fragments and recombinant proteins.
Contact AtaGenixContact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

