AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-10-15 View volume: 15
In the complex gut microbial community, competition among bacterial populations determines ecological niche stability and dominance. The Type VI Secretion System (T6SS) is a widespread secretion apparatus in Gram-negative bacteria, capable of injecting toxic effector proteins into competing bacteria in a contact-dependent manner, enabling "kin-killing" and ecological dominance. Although previous studies have shown that T6SS can deliver various effector molecules, whether these effectors can be co-loaded in a single secretion event, how they are recognized and released, and how they achieve functional synergy have long remained unclear. This study uses Bacteroides fragilis GS086 as a model to reveal the complete process of co-secretion of two effector proteins, BtpeA and BtaeB, coordinated by a novel adaptor protein, BtapC. It resolves the cryo-EM structure of the quaternary complex VgrG–BtpeA–BtaeB–BtapC and elucidates its functional significance in gut microbiota competition. This discovery not only uncovers a unique evolutionary pattern of the T6SS system in Bacteroidota but also provides a new theoretical framework for understanding multi-effector co-secretion.
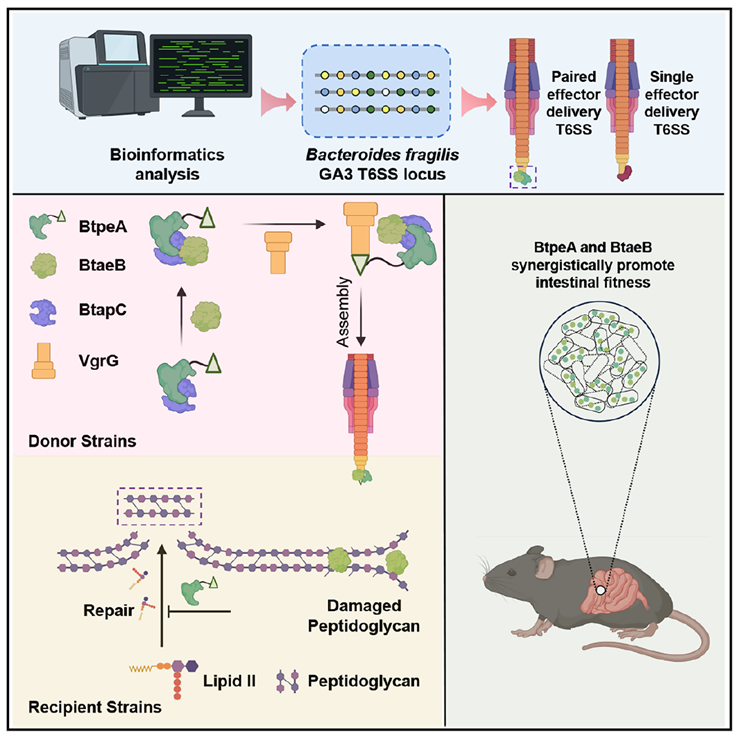
Figure 1. Graphical abstract
T6SS V2 Locus in B. fragilis GS086 Carries Two Effectors Critical for Interspecies Competition
The study began with a genomic comparison of the T6SS V2 locus in B. fragilis GS086, identifying two potential effector genes, btpeA and btaeB, and an unannotated gene, btapC. Wild-type strains exhibited strong killing activity when co-cultured with the recipient strain Bacteroides xylanisolvens, whereas deletion of btpeA, btaeB, or btapC completely abolished this killing effect, similar to T6SS-inactivated mutants. This suggests a functional dependency among the three. Further immunoblotting experiments showed that secretion of BtpeA and BtaeB depends on the T6SS core component Hcp, and their overexpression in the recipient bacterium’s periplasm caused significant cell swelling and lysis. These assays utilized rabbit polyclonal antibodies (anti-BtpeA, anti-BtaeB, anti-BtapC, anti-Hcp1) provided by AtaGenix. Western blot analysis, using the cytoplasmic protein DnaK as an internal control, clearly distinguished the distribution of target proteins between whole-cell lysates and culture supernatants, confirming the T6SS-dependent secretion of BtpeA and BtaeB.
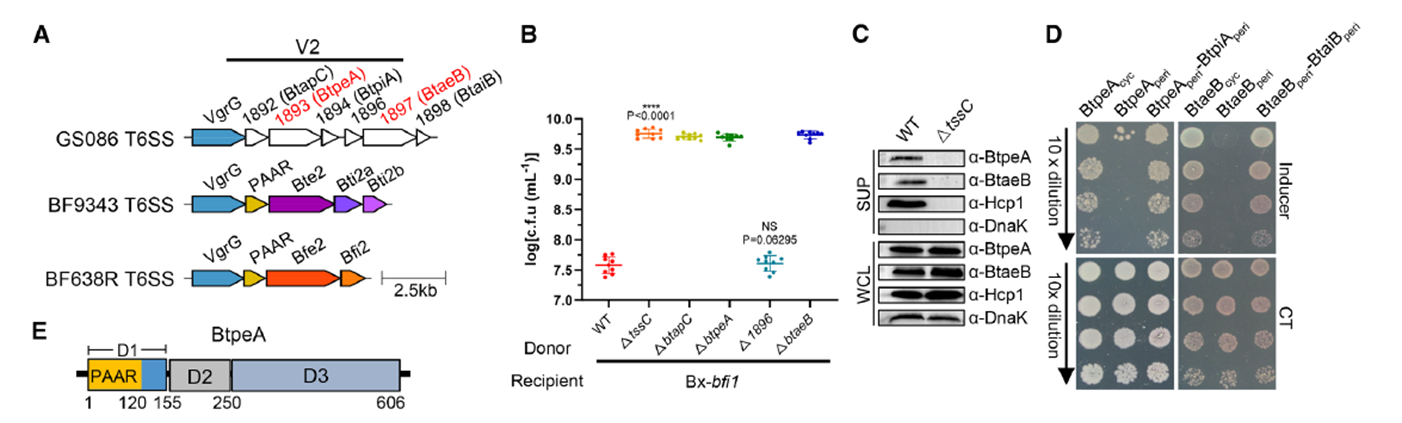
Figure 2. Detection of Two Adjacent T6SS Effector Loci
BtpeA and BtaeB Are Functionally Distinct Enzymes That Disrupt Cell Walls
Structural and biochemical analyses further revealed the functional characteristics of the two effector proteins. BtpeA contains a PAAR domain and two unannotated functional domains, with its C-terminal D3 domain showing high homology to Escherichia coli ColM phosphatase, catalyzing the dephosphorylation of the cell wall precursor molecule Lipid II, thereby blocking peptidoglycan synthesis. BtaeB, on the other hand, comprises an N-terminal domain and a C-terminal amidase domain belonging to the C39 family, cleaving peptidoglycan tetrapeptide cross-links via active sites Cys460 and His546, directly disrupting the cell wall. These two effectors attack cell wall synthesis and degradation from complementary angles. The study also found significant mutual dependence in the secretion of BtpeA and BtaeB: deletion of one effector gene completely inhibited the secretion of the other, although their intracellular expression levels remained unaffected. This interdependence suggests they are loaded onto the T6SS VgrG apparatus as a single unit.
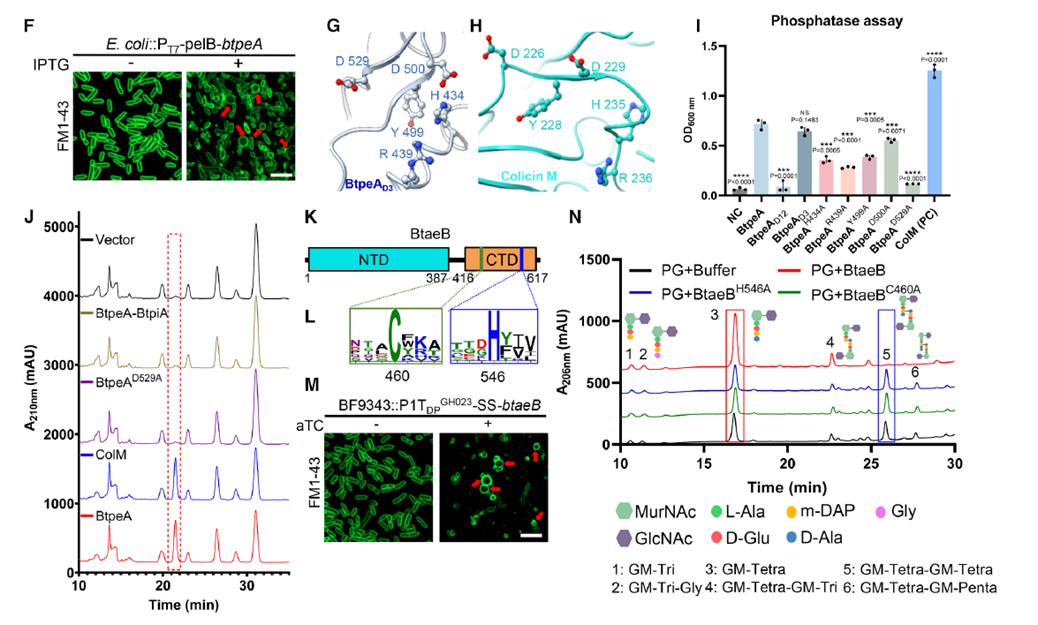
Figure 3. Cell Wall-Damaging T6SS Effectors Are Critical for Interspecies Competition
T6SS-Mediated Secretion of BtpeA and BtaeB Is Mutually Dependent
To uncover the molecular basis of this co-secretion, researchers focused on BtapC, located in the same gene cluster. Deletion of btapC not only abolished BtpeA and BtaeB secretion but also reduced Hcp release, indicating BtapC’s central role in T6SS assembly. Pull-down and co-immunoprecipitation experiments revealed that BtapC directly binds BtpeA to form a binary complex, with BtaeB recruited only after this complex forms, generating a ternary complex BtpeA–BtaeB–BtapC. Further experiments showed this ternary complex binds VgrG to form a stable quaternary loading complex VgrG–BtpeA–BtaeB–BtapC. These results, validated both in vivo and in vitro, confirmed BtapC’s critical role as a “loading adaptor.” The AtaGenix anti-BtapC antibody successfully detected BtapC in the complex, and together with anti-BtpeA, anti-BtaeB, and anti-VgrG antibodies, clarified BtapC’s bridging role in complex assembly.
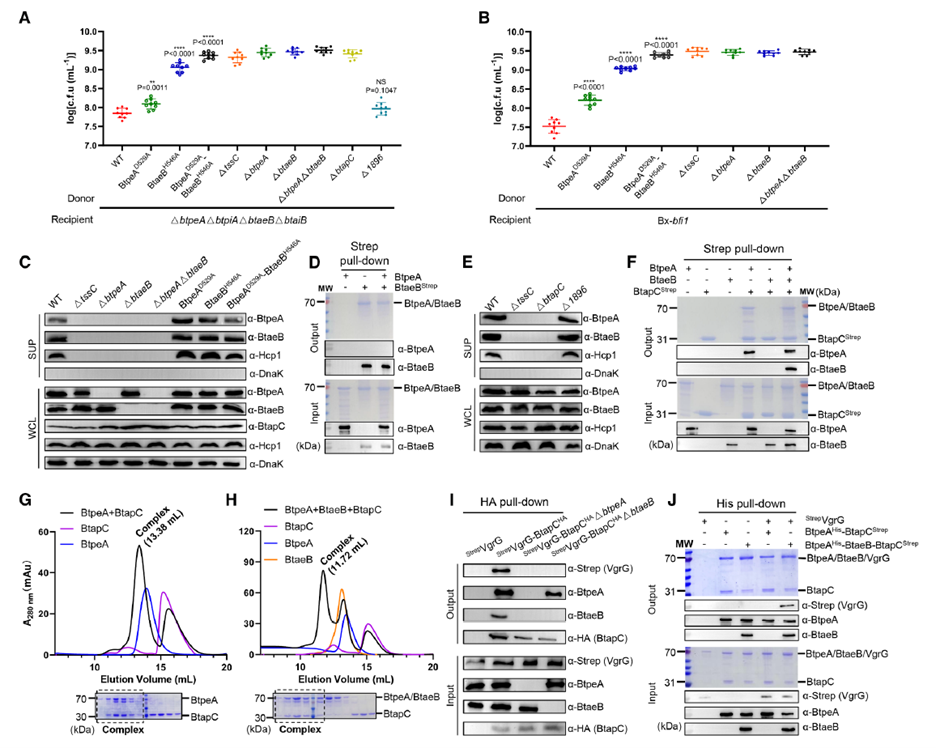
Figure 4. Ternary Complex Formation Coordinated by BtapC and Subsequently Loaded onto VgrG
Cryo-EM Structure and Assembly Mechanism of the VgrG–BtpeA–BtaeB–BtapC Quaternary Complex
Single-particle cryo-EM analysis resolved the quaternary complex at a 3.06 Å resolution, revealing a complex and precise molecular assembly. BtapC adopts an open clamshell-like conformation, with its structural cavity tightly enveloping the N-terminal α1–α4 helices of BtpeA, stabilized by extensive hydrophobic and polar interactions. This structure not only establishes the BtpeA–BtapC binding interface but also provides spatial support for BtaeB loading. Upon binding the BtpeA–BtapC complex, BtaeB’s β12 segment undergoes a significant conformational change, enabling the Lys188 side chain to form hydrogen bonds with both BtpeA’s Asp148 and BtapC’s Ser127, creating a stable interaction network among the three. Point mutation experiments confirmed the critical role of these amino acid residues in complex formation. Notably, BtpeA’s PAAR domain is loaded onto the VgrG tip, with BtaeB’s presence being essential for maintaining the stability of this process. AtaGenix anti-BtpeA and anti-BtapC antibodies accurately identified truncated protein variants, providing specific detection tools for validating structure-function relationships.
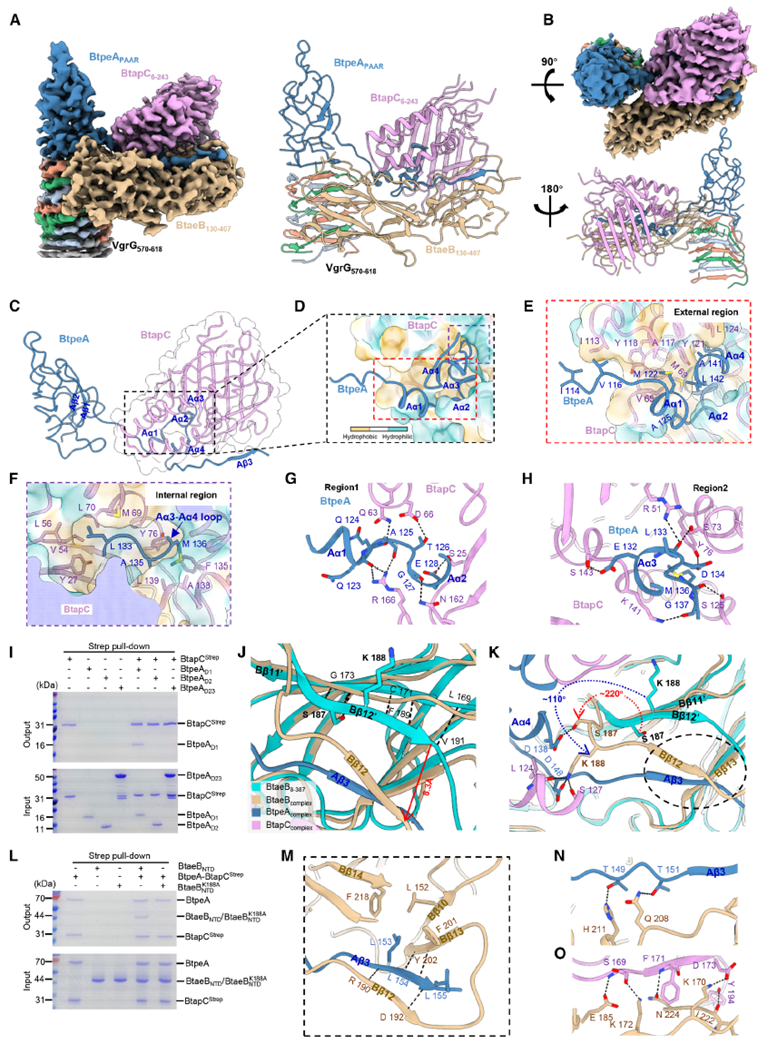
Figure 5. Structure of the VgrG–BtpeA–BtaeB–BtapC Complex
BtaeB’s Conserved Loop Structure Is Critical for BtpeA and BtaeB Co-Secretion
Further structural analysis identified a unique short loop (residues 400–407) at BtaeB’s C-terminus, with its electron density located at the VgrG–BtpeA PAAR interface. This loop embeds into the triangular region between VgrG and BtpeA, forming multiple hydrogen bonds and hydrophobic interactions, acting as a “lock and key” to stabilize the complex. Functional validation showed that BtaeB mutants retaining this loop could secrete BtpeA and Hcp normally, while loop deletion completely abolished secretion. This confirms that the short loop serves as a structural checkpoint for the coordinated loading of dual effectors, ensuring both effector molecules are synchronously injected into the recipient bacterium during each T6SS firing event.
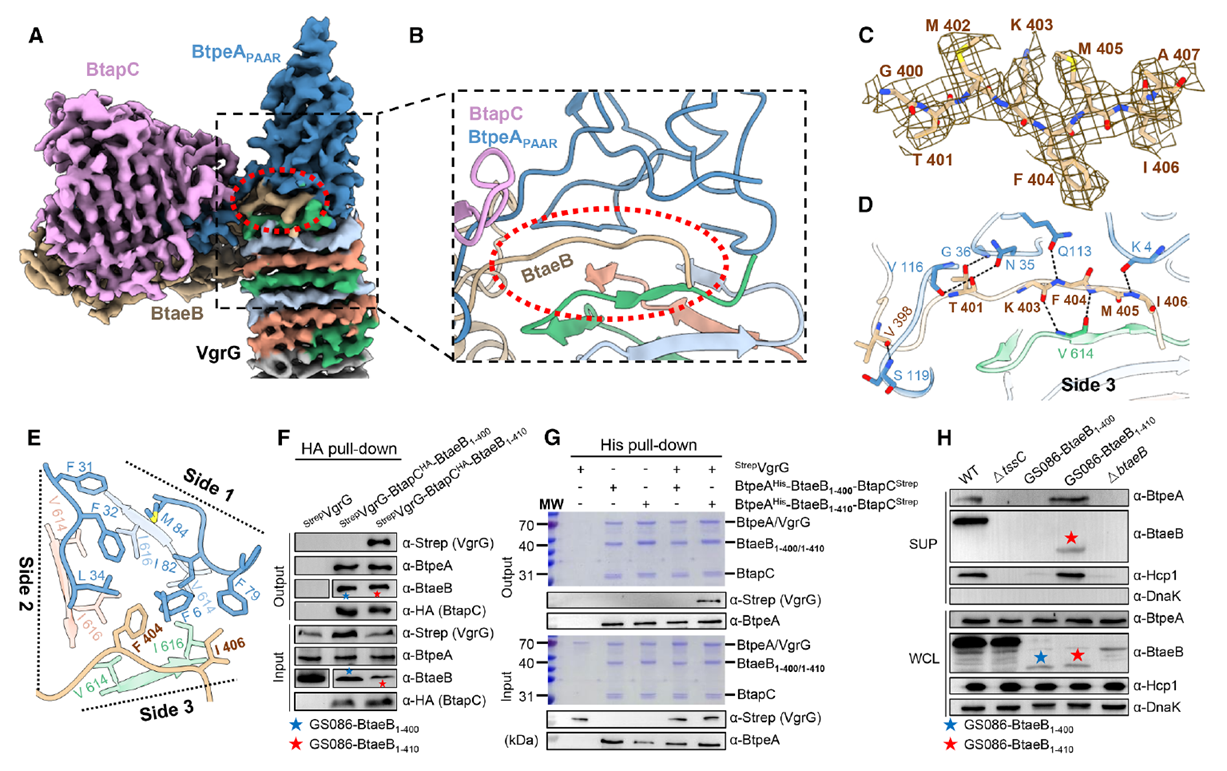
Figure 6. BtaeB-Derived Loop Is Critical for BtpeA and BtaeB Co-Secretion
Co-Secreted BtpeA and BtaeB Synergistically Enhance B. fragilis Gut Fitness
Animal experiments further validated the physiological significance of this co-secretion in ecological competition. Wild-type and mutant strains were co-colonized with sensitive strains in the guts of antibiotic-treated germ-free mice. Results showed that inactivating BtpeA alone reduced competitiveness against the recipient by approximately 10³-fold, while inactivating BtaeB alone or completely lacking T6SS nearly abolished competitive ability. In contrast, wild-type strains reduced recipient abundance by about 10⁵-fold, demonstrating significant synergistic toxicity of BtpeA and BtaeB in the gut environment. Specifically, BtpeA blocks cell wall repair, while BtaeB directly cleaves peptidoglycan cross-links, together causing irreversible cell wall collapse and conferring a stronger gut colonization advantage. Bioinformatics analysis revealed that this BtapC-mediated dual-effector co-secretion mechanism is widespread in Bacteroidota, with approximately 50% of B. fragilis strains carrying similar structural modules, suggesting high conservation and adaptability in gut bacterial evolution.
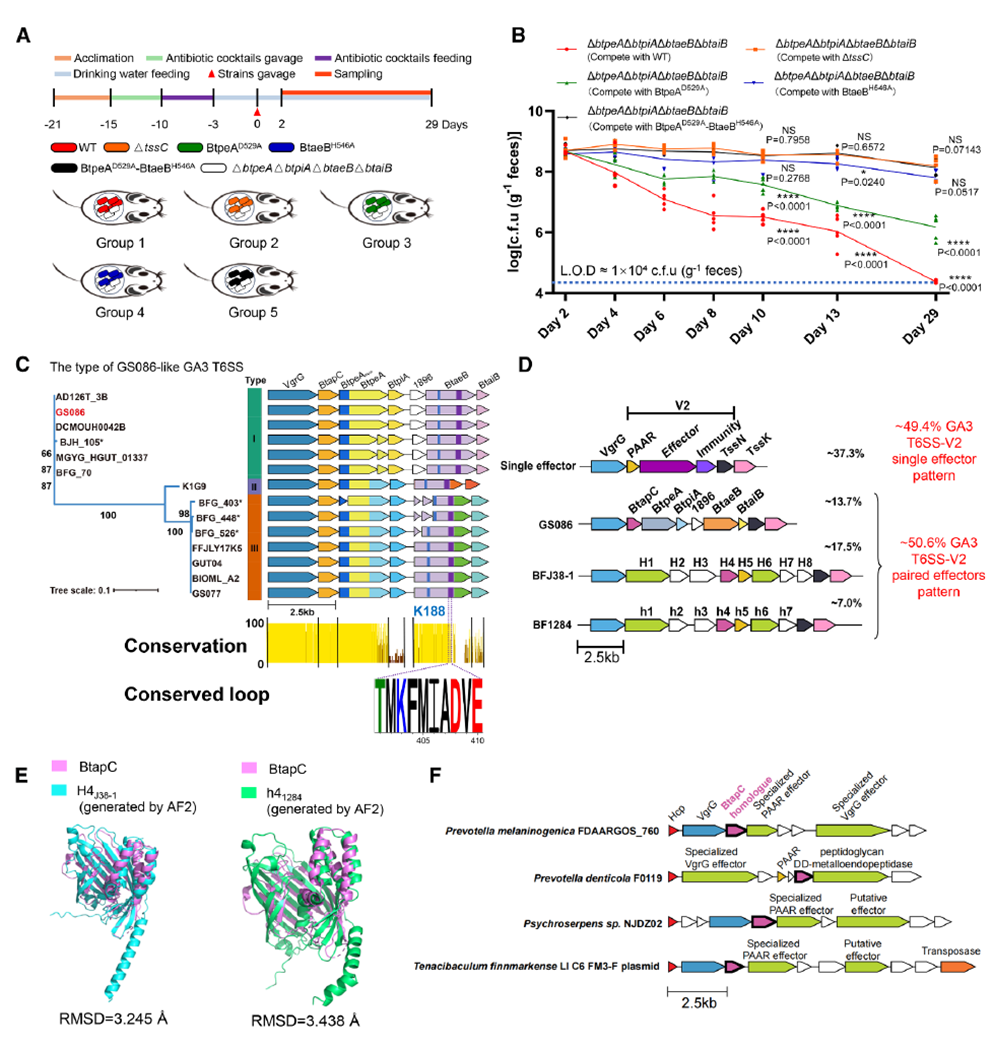
Figure 7. BtapC-Mediated Effector Co-Secretion Produces Functional Synergy in Mice and Is Likely Conserved in Human Gut Bacteroidota
Overall, this study is the first to systematically reveal a unique T6SS co-secretion mode in gut commensal bacteria: BtapC, as a core adaptor, forms an initial complex with BtpeA and sequentially recruits BtaeB, enabling two structurally and functionally complementary effector proteins to be co-injected into competing bacteria in a single T6SS firing event. The short loop at BtaeB’s C-terminus inserts into the VgrG–BtpeA interface, forming a “lock and key” that ensures stable assembly and synchronous secretion of the quaternary complex. This mechanism not only achieves multi-effector synergy at the structural level but also significantly enhances the strain’s competitiveness in the gut ecosystem through functional complementation. Notably, this discovery suggests that T6SS may possess “modular assembly” potential, combining conserved loading scaffolds with variable toxic modules to achieve targeted adaptation to different environments. Using AtaGenix polyclonal antibodies (anti-BtpeA, anti-BtaeB, anti-BtapC, anti-Hcp1), researchers precisely tracked complex formation and secretion, providing robust tools for T6SS structure-function studies. In the future, similar loading strategies could be engineered for developing microbiome-targeted interventions or designing novel precision antibacterial systems against drug-resistant bacteria.
As of October 2025, over 400 publications have cited AtaGenix’s one-stop protein and antibody development services. Looking ahead, AtaGenix will continue to support scientific innovation.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

