AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2025-10-14 View volume: 46
Published on October 14, 2025
October 14, 2025: The biopharmaceutical industry is undergoing a transformative shift as AI-driven antibody design compresses development cycles from months or years to mere weeks, delivering up to 100-fold efficiency improvements. By leveraging DeepMind’s AlphaFold, AtaGenix Laboratories addresses the complexity of antibody CDR regions to generate high-affinity candidates (Kd < 1 nM). Paired with its robust wet-lab validation, AtaGenix mitigates risks of over-fitting on limited datasets (e.g., SARS-CoV-2 data) and empowers clients to tackle high-value targets like GPCR and PD-L1, redefining the economics of drug discovery.
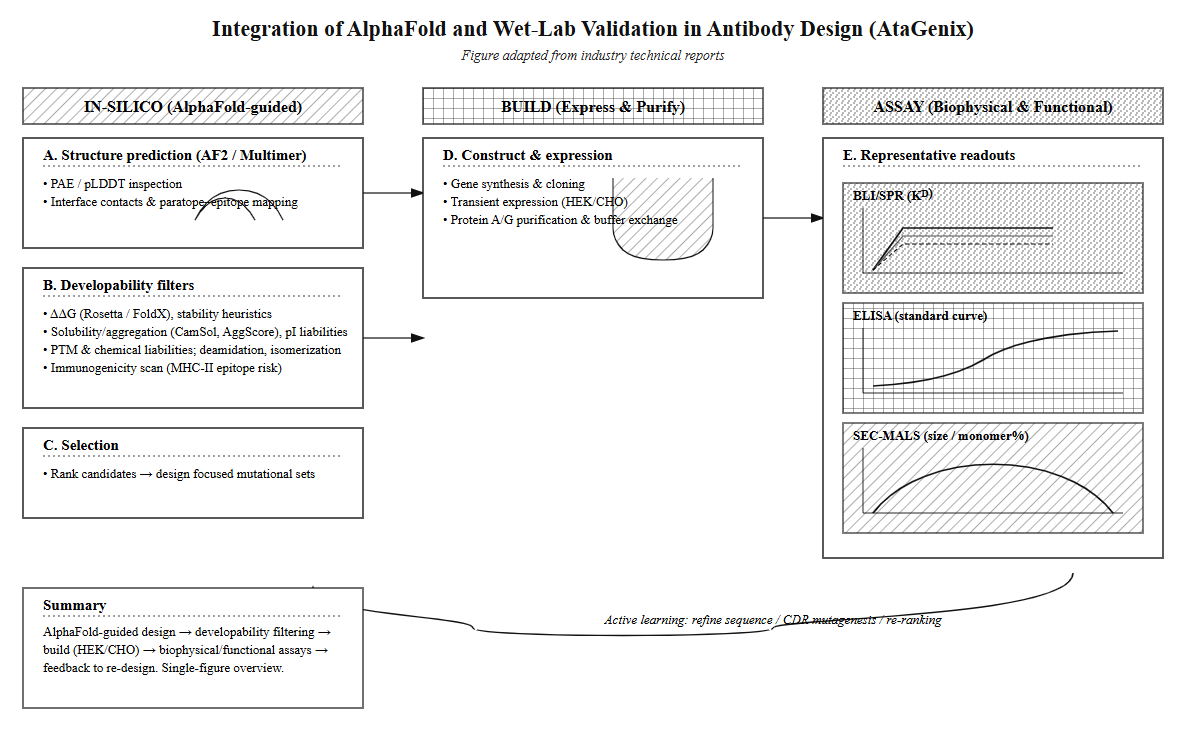
Antibody design faces unique challenges due to the hypervariable CDR regions, which can span 20+ amino acids and resist traditional methods like evolutionary algorithms or early folding predictions. Since 2024, AI tools have revolutionized this space. David Baker's RFantibody, a diffusion model, generates structures by denoising from random noise while respecting immunoglobulin frameworks, though its stochastic nature demands thousands of trials, escalating validation costs.
Building on this, hybrid models from global leaders like Generate Biomedicines and China's Helixon blend diffusion with antibody language models trained on millions of sequences, filtering implausible designs to enhance success rates. These models optimize CDR sequences for structural compatibility and binding affinity. A notable advancement is Chai-2, released in June 2025, which achieves zero-shot de novo antibody design with a 16-20% experimental hit rate—over 100 times better than traditional methods' 0.1%. Chai-2 uses a multimodal generative framework, combining all-atom diffusion models with sequence optimization to design all CDRs from scratch, prompted solely by target structure and epitope, producing nanomolar-affinity binders in two weeks across 52 novel targets (50% success rate). It supports formats like scFv, VHH nanobodies, and miniproteins (68% hit rate), including cross-reactive human-cyno variants and picomolar-affinity TNFα binders.
| Axis | Current Emphasis and Metrics |
|---|---|
| By Modality | AI-driven mAbs (40% timeline reduction, 8/10 binding success for PD-L1); multispecifics (50% affinity improvement); nanobodies (7/10 success for IL7Ra, low nM Kd) |
| By Approach | AI design with AlphaFold (100x efficiency, weeks vs. months); phage display (4-6 weeks screening); B-cell cloning (natural low-immunogenicity hits); stable cell lines (>3 g/L yield, AI-optimized) |
| By Region and Barriers | Asia-Pacific (25% CAGR, data flywheels via collaborations); North America (55% market, GPCR focus); Europe (regulatory hurdles); Global challenge: Data scarcity (<50k affinity datapoints, viral bias) |
The primary bottleneck in AI antibody design is not algorithms but data scarcity. Public datasets like AbRank (~50k points, skewed toward SARS-CoV-2/HIV) limit generalization. AtaGenix tackles this by building proprietary data flywheels: Client projects generate validated affinity data, refining AI models iteratively. Using AlphaFold for structural validation, AtaGenix ensures designs are both foldable and functional, reducing false positives through molecular dynamics simulations. In 2025's booming AI-biotech market, trends emphasize programmable engineering for bispecifics and ADCs, but validation remains critical for clinical translation.
Commercially, AtaGenix leverages AlphaFold to accelerate antibody development, enabling rapid target feasibility testing at a fraction of traditional costs (e.g., <5% of millions spent). This approach allows clients, including multinational corporations (MNCs) and biotech startups, to shift focus from discovery to clinical execution and disease biology understanding, meeting the rising demand for differentiated R&D in a competitive landscape.
The AI-driven antibody discovery era, powered by tools like AlphaFold, signals a future where 99% of designs could be computationally generated, but success hinges on high-quality data and rigorous validation. AtaGenix transforms traditional CRO models into data-centric powerhouses by integrating AlphaFold with its core services:
Our approach slashes timelines by over 50% and costs to <5% of traditional methods, addressing over-fitting through diverse dataset curation. For long-tail targets, AtaGenix's AlphaFold-driven frameworks enable customized optimization, ensuring clients navigate AI's paradox: abundant designs, rare breakthroughs.
Note: Data aggregated from professional reports, competitions like AIntibody, and AtaGenix's proprietary insights.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

